 “Breast cancer (BC) is the most common cancer in women worldwide. Approximately 70-80% of BCs express estrogen receptors (ER), which predict the response to endocrine therapy (ET), and are therefore hormone receptor-positive (HR+).
“Breast cancer (BC) is the most common cancer in women worldwide. Approximately 70-80% of BCs express estrogen receptors (ER), which predict the response to endocrine therapy (ET), and are therefore hormone receptor-positive (HR+).
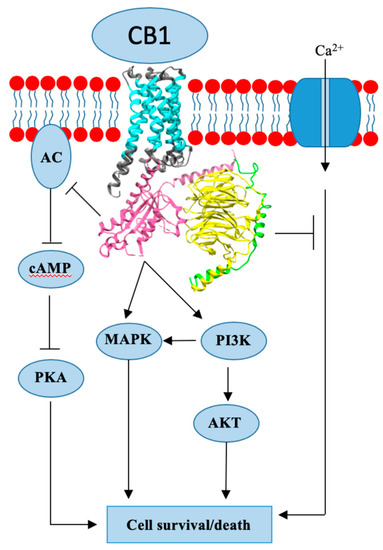
Endogenous cannabinoids together with cannabinoid receptor 1 and 2 (CB1, CB2) constitute the basis of the endocannabinoid system.
Interactions of cannabinoids with hypothalamic-pituitary-gonadal axis hormones are well documented, and two studies found a positive correlation between peak plasma endogenous cannabinoid anandamide with peak plasma 17β-estradiol, luteinizing hormone and follicle-stimulating hormone levels at ovulation in healthy premenopausal women. Do cannabinoids have an effect on HR+ BC? In this paper we review known and possible interactions between cannabinoids and specific HR+ BC treatments.
In preclinical studies, CB1 and CB2 agonists (i.e., anandamide, THC) have been shown to inhibit the proliferation of ER positive BC cell lines.
There is less evidence for antitumor cannabinoid action in HR+ BC in animal models and there are no clinical trials exploring the effects of cannabinoids on HR+ BC treatment outcomes. Two studies have shown that tamoxifen and several other selective estrogen receptor modulators (SERM) can act as inverse agonists on CB1 and CB2, an interaction with possible clinical consequences. In addition, cannabinoid action could interact with other commonly used endocrine and targeted therapies used in the treatment of HR+ BC.”
https://www.ncbi.nlm.nih.gov/pubmed/32106399
https://www.mdpi.com/2072-6694/12/3/525

 “Cannabinoids are a group of structurally heterogeneous but pharmacologically related compounds, including plant-derived cannabinoids, synthetic substances and endogenous cannabinoids, such as anandamide and 2-arachidonoylglycerol.
“Cannabinoids are a group of structurally heterogeneous but pharmacologically related compounds, including plant-derived cannabinoids, synthetic substances and endogenous cannabinoids, such as anandamide and 2-arachidonoylglycerol. “Anticholinergic organophosphate (OP) agents act on the diverse serine hydrolases, thereby revealing unexpected biological effects. Epidemiological studies indicate a relationship between OP exposure and development of attention-deficit/hyperactivity disorder (ADHD)-like symptoms, whereas no plausible mechanism for the OP-induced ADHD has been established.
“Anticholinergic organophosphate (OP) agents act on the diverse serine hydrolases, thereby revealing unexpected biological effects. Epidemiological studies indicate a relationship between OP exposure and development of attention-deficit/hyperactivity disorder (ADHD)-like symptoms, whereas no plausible mechanism for the OP-induced ADHD has been established. “Stress is a risk factor for psychosis and treatments which mitigate its harmful effects are needed.
“Stress is a risk factor for psychosis and treatments which mitigate its harmful effects are needed. “This review focuses on the possible roles of phytocannabinoids, synthetic cannabinoids, endocannabinoids, and “transient receptor potential cation channel, subfamily V, member 1” (TRPV1) channel blockers in epilepsy treatment.
“This review focuses on the possible roles of phytocannabinoids, synthetic cannabinoids, endocannabinoids, and “transient receptor potential cation channel, subfamily V, member 1” (TRPV1) channel blockers in epilepsy treatment. “Excessive binge alcohol drinking may adversely affect cardiovascular function. In this study we characterize the detailed hemodynamic effects of an acute alcohol binge in mice using multiple approaches and investigate the role of the endocannabinoid-
“Excessive binge alcohol drinking may adversely affect cardiovascular function. In this study we characterize the detailed hemodynamic effects of an acute alcohol binge in mice using multiple approaches and investigate the role of the endocannabinoid-
 “Endocannabinoids are produced within the gastrointestinal (GI) tract and modulate energy homeostasis and food intake, at least in part, via vagally-dependent actions. The recent paper by Christie et al., [Christie, et al. J Physiol, 2019] demonstrate, for the first time, that
“Endocannabinoids are produced within the gastrointestinal (GI) tract and modulate energy homeostasis and food intake, at least in part, via vagally-dependent actions. The recent paper by Christie et al., [Christie, et al. J Physiol, 2019] demonstrate, for the first time, that 

 “Brain trauma was clinically associated with increased osteogenesis in the appendicular skeleton. We showed previously in C57BL/6J mice that mild traumatic brain injury (mTBI) transiently induced bone formation in the femur via the
“Brain trauma was clinically associated with increased osteogenesis in the appendicular skeleton. We showed previously in C57BL/6J mice that mild traumatic brain injury (mTBI) transiently induced bone formation in the femur via the  “Preclinical studies have shown that cannabidiol (CBD) mitigates fear memories by facilitating their extinction or interfering with their generalization and reconsolidation. The brain regions and mechanisms underlying these effects, and their temporal window, are still poorly understood. The present paper aimed at investigating related questions in the dorsal hippocampus (DH) during contextual fear consolidation.
“Preclinical studies have shown that cannabidiol (CBD) mitigates fear memories by facilitating their extinction or interfering with their generalization and reconsolidation. The brain regions and mechanisms underlying these effects, and their temporal window, are still poorly understood. The present paper aimed at investigating related questions in the dorsal hippocampus (DH) during contextual fear consolidation.