 “Precise cannabis treatment dosing remains a major challenge, leading to physicians’ reluctance to prescribe medical cannabis.
“Precise cannabis treatment dosing remains a major challenge, leading to physicians’ reluctance to prescribe medical cannabis.
Objective
To test the pharmacokinetics, analgesic effect, cognitive performance and safety effects of an innovative medical device that enables the delivery of inhaled therapeutic doses of Δ9‐Tetrahydrocannabinol (THC) in patients with chronic pain.
Methods
In a randomized, three‐arms, double‐blinded, placebo‐controlled, cross‐over trial, 27 patients received a single inhalation of Δ9‐THC: 0.5mg, 1mg, or a placebo.
Δ9‐THC plasma levels were measured at baseline and up to 150‐min post‐inhalation. Pain intensity and safety parameters were recorded on a 10‐cm visual analogue scale (VAS) at pre‐defined time points. The cognitive performance was evaluated using the selective sub‐tests of the Cambridge Neuropsychological Test Automated Battery (CANTAB).
Results
Following inhalation of 0.5 mg or 1mg, Δ9‐THC plasma C max ± SD were 14.3 ± 7.7 and 33.8 ± 25.7 ng/ml. T max ± SD were 3.7 ± 1.4 and 4.4 ± 2.1 min, and AUC0 → infinity±SD were 300 ± 144 and 769 ± 331 ng*min/ml, respectively. Both doses, but not the placebo, demonstrated a significant reduction in pain intensity compared with baseline and remained stable for 150‐min. The 1‐mg dose showed a significant pain decrease compared to the placebo. Adverse events were mostly mild and resolved spontaneously. There was no evidence of consistent impairments in cognitive performance.
Conclusion
This feasibility trial demonstrated that a metered‐dose cannabis inhaler delivered precise and low THC doses, produced a dose‐dependent and safe analgesic effect in patients with neuropathic pain/ complex‐regional pain syndrome (CRPS). Thus, it enables individualization of medical cannabis regimens that can be evaluated pharmacokinetically and pharmacodynamically by accepted pharmaceutical models.
Significance
Evidence suggests that cannabis‐based medicines are an effective treatment for chronic pain in adults. The pharmacokinetics of THC varies as a function of its route of administration. Pulmonary assimilation of inhaled THC causes rapid onset of analgesia. However, currently used routes of cannabinoids delivery provide unknown doses, making it impossible to implement a pharmaceutical standard treatment plan. A novel selective‐dose cannabis inhaler delivers significantly low and precise doses of THC, thus allowing the administration of inhaled cannabis‐based medicines according to high pharmaceutical standards. These low doses of THC can produce safe and effective analgesia in patients with chronic pain.
To the best of our knowledge, it is the first time that the delivery of selective, significantly low, and precise therapeutic single doses of inhaled THC demonstrates an analgesic effect. It allows patients to reach the optimum balance between symptom relief and controlled side effects, enabling patients to regain their quality of life. In addition, this metered‐dose cannabis inhaler enables the individualization of medical cannabis regimens that can be evaluated pharmacokinetically and pharmacodynamically using accepted pharmaceutical models.”
https://onlinelibrary.wiley.com/doi/10.1002/ejp.1605

 “Extracts from the cannabis plant can dramatically improve the health of children suffering from refractory epilepsies such as Dravet syndrome.
“Extracts from the cannabis plant can dramatically improve the health of children suffering from refractory epilepsies such as Dravet syndrome. “Scientific research on how consumption of whole, natural Cannabis flower affects low mood and behavioral motivations more generally is largely nonexistent, and few studies to date have measured how common and commercially available Cannabis flower used in vivo may affect the experience of “depression” in real-time.
“Scientific research on how consumption of whole, natural Cannabis flower affects low mood and behavioral motivations more generally is largely nonexistent, and few studies to date have measured how common and commercially available Cannabis flower used in vivo may affect the experience of “depression” in real-time. “Genus Cannabis belong to family Cannabaceae and is traditionally used as medicinal plant against many diseases notably asthma, malaria, treatment of skin diseases, diabetes and headache. The plant Cannabis sativa L. is flowering and an annual herbaceous plant located to eastern Asia but now of cosmopolitan distribution due to extensive cultivation.
“Genus Cannabis belong to family Cannabaceae and is traditionally used as medicinal plant against many diseases notably asthma, malaria, treatment of skin diseases, diabetes and headache. The plant Cannabis sativa L. is flowering and an annual herbaceous plant located to eastern Asia but now of cosmopolitan distribution due to extensive cultivation. “Studies have reported changes in the endocannabinoid system in the brain of patients with Alzheimer’s disease (AD), playing a role in the pathophysiology of AD. Cannabinoids have been shown to have neuroprotective properties, reduce neuroinflammation, and enhance neurogenesis. Evidence suggests that the utilization of marijuana products containing both tetrahydrocannabinol (THC) and cannabidiol (CBD) or CBD alone have been effective and safe for use in older people with agitation associated with dementia.
“Studies have reported changes in the endocannabinoid system in the brain of patients with Alzheimer’s disease (AD), playing a role in the pathophysiology of AD. Cannabinoids have been shown to have neuroprotective properties, reduce neuroinflammation, and enhance neurogenesis. Evidence suggests that the utilization of marijuana products containing both tetrahydrocannabinol (THC) and cannabidiol (CBD) or CBD alone have been effective and safe for use in older people with agitation associated with dementia. “The seed of the hemp plant (Cannabis sativa L.) has been revered as a nutritional resource in Old World Cultures. This has been confirmed by contemporary science wherein hempseed oil (HSO) was found to exhibit a desirable ratio of omega-6 and omega-3 polyunsaturated fatty acids (PUFAs) considered optimal for human nutrition. HSO also contains gamma-linoleic acid (GLA) and non-psychoactive cannabinoids, which further contribute to its’ potential bioactive properties. Herein, we present the kinetics of the thermal stability of these nutraceutical compounds in HSO, in the presence of various antioxidants (e.g. butylated hydroxytoluene, alpha-tocopherol, and ascorbyl palmitate). We focussed on oxidative changes in fatty acid profile and acidic cannabinoid stability when HSO was heated at different temperatures (25 °C to 85 °C) for upto 24 h. The fatty acid composition was evaluated using both GC/MS and 1H-NMR, and the cannabinoids profile of HSO was obtained using both HPLC-UV and HPLC/MS methods. The predicted half-life (DT50) for omega-6 and omega-3 PUFAs in HSO at 25 °C was about 3 and 5 days, respectively; while that at 85 °C was about 7 and 5 hours respectively, with respective activation energies (Ea) being 54.78 ± 2.36 and 45.02 ± 2.87 kJ/mol. Analysis of the conjugated diene hydroperoxides (CDH) and p-Anisidine value (p-AV) revealed that the addition of antioxidants significantly (p < 0.05) limited lipid peroxidation of HSO in samples incubated at 25-85 °C for 24 h. Antioxidants reduced the degradation constant (k) of PUFAs in HSO by upto 79%. This corresponded to a significant (p < 0.05) increase in color stability and pigment retention (chlorophyll a, chlorophyll b and carotenoids) of heated HSO. Regarding the decarboxylation kinetics of cannabidiolic acid (CBDA) in HSO, at both 70 °C and 85 °C, CBDA decarboxylation led to predominantly cannabidiol (CBD) production. The half-life of CBDA decarboxylation (originally 4 days) could be increased to about 17 days using tocopherol as an antioxidant. We propose that determining acidic cannabinoids decarboxylation kinetics is a useful marker to measure the shelf-life of HSO. The results from the study will be useful for researchers looking into the thermal treatment of hempseed oil as a functional food product, and those interested in the decarboxylation kinetics of the acidic cannabinoids.”
“The seed of the hemp plant (Cannabis sativa L.) has been revered as a nutritional resource in Old World Cultures. This has been confirmed by contemporary science wherein hempseed oil (HSO) was found to exhibit a desirable ratio of omega-6 and omega-3 polyunsaturated fatty acids (PUFAs) considered optimal for human nutrition. HSO also contains gamma-linoleic acid (GLA) and non-psychoactive cannabinoids, which further contribute to its’ potential bioactive properties. Herein, we present the kinetics of the thermal stability of these nutraceutical compounds in HSO, in the presence of various antioxidants (e.g. butylated hydroxytoluene, alpha-tocopherol, and ascorbyl palmitate). We focussed on oxidative changes in fatty acid profile and acidic cannabinoid stability when HSO was heated at different temperatures (25 °C to 85 °C) for upto 24 h. The fatty acid composition was evaluated using both GC/MS and 1H-NMR, and the cannabinoids profile of HSO was obtained using both HPLC-UV and HPLC/MS methods. The predicted half-life (DT50) for omega-6 and omega-3 PUFAs in HSO at 25 °C was about 3 and 5 days, respectively; while that at 85 °C was about 7 and 5 hours respectively, with respective activation energies (Ea) being 54.78 ± 2.36 and 45.02 ± 2.87 kJ/mol. Analysis of the conjugated diene hydroperoxides (CDH) and p-Anisidine value (p-AV) revealed that the addition of antioxidants significantly (p < 0.05) limited lipid peroxidation of HSO in samples incubated at 25-85 °C for 24 h. Antioxidants reduced the degradation constant (k) of PUFAs in HSO by upto 79%. This corresponded to a significant (p < 0.05) increase in color stability and pigment retention (chlorophyll a, chlorophyll b and carotenoids) of heated HSO. Regarding the decarboxylation kinetics of cannabidiolic acid (CBDA) in HSO, at both 70 °C and 85 °C, CBDA decarboxylation led to predominantly cannabidiol (CBD) production. The half-life of CBDA decarboxylation (originally 4 days) could be increased to about 17 days using tocopherol as an antioxidant. We propose that determining acidic cannabinoids decarboxylation kinetics is a useful marker to measure the shelf-life of HSO. The results from the study will be useful for researchers looking into the thermal treatment of hempseed oil as a functional food product, and those interested in the decarboxylation kinetics of the acidic cannabinoids.” “The efficacy of cannabidiol (CBD) with and without concomitant clobazam (CLB) was evaluated in stratified analyses of four large randomized controlled trials, two in Lennox-Gastaut syndrome and two in Dravet syndrome.
“The efficacy of cannabidiol (CBD) with and without concomitant clobazam (CLB) was evaluated in stratified analyses of four large randomized controlled trials, two in Lennox-Gastaut syndrome and two in Dravet syndrome. “The Cannabis plant contains numerous components, including cannabinoids and other active molecules. The phyto-cannabinoid activity is mediated by the endocannabinoid system. Cannabinoids affect the nervous system and play significant roles in the regulation of the immune system.
“The Cannabis plant contains numerous components, including cannabinoids and other active molecules. The phyto-cannabinoid activity is mediated by the endocannabinoid system. Cannabinoids affect the nervous system and play significant roles in the regulation of the immune system.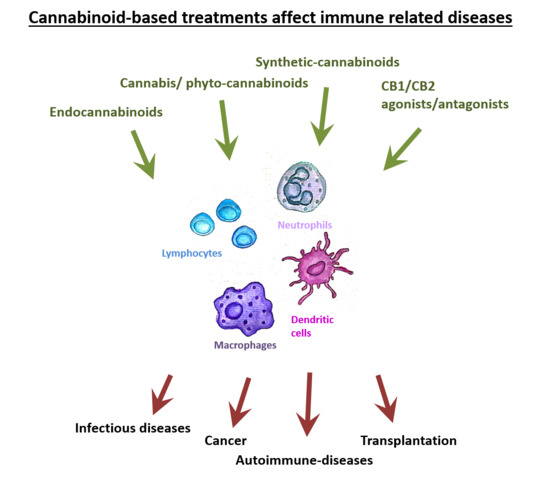
 “Current antiepileptic drugs (AEDs) are undesirable for many reasons including the inability to reduce seizures in certain types of epilepsy, such as Dravet syndrome (DS) where in one-third of patients does not respond to current AEDs, and severe adverse effects that are frequently experienced by patients.
“Current antiepileptic drugs (AEDs) are undesirable for many reasons including the inability to reduce seizures in certain types of epilepsy, such as Dravet syndrome (DS) where in one-third of patients does not respond to current AEDs, and severe adverse effects that are frequently experienced by patients. “Objective: To determine whether cannabis may reduce HIV-related persistent inflammation, we evaluated the relationship of cannabis use in people with HIV (PWH) to inflammatory cytokines in CSF and blood plasma.
“Objective: To determine whether cannabis may reduce HIV-related persistent inflammation, we evaluated the relationship of cannabis use in people with HIV (PWH) to inflammatory cytokines in CSF and blood plasma.