
CONCLUSIONS:
The results of this study suggest adjunctive dronabinol reduces opioid consumption following traumatic injury.
The opioid-sparing effect of dronabinol may be greater in patients who are marijuana users.”


The results of this study suggest adjunctive dronabinol reduces opioid consumption following traumatic injury.
The opioid-sparing effect of dronabinol may be greater in patients who are marijuana users.”
 “Excessive fear and anxiety, coupled with corticolimbic dysfunction, are core features of stress- and trauma-related psychopathology, such as posttraumatic stress disorder (PTSD).
“Excessive fear and anxiety, coupled with corticolimbic dysfunction, are core features of stress- and trauma-related psychopathology, such as posttraumatic stress disorder (PTSD).
Interestingly, low doses of ∆9-tetrahydrocannabinol (THC) can produce anxiolytic effects, reduce threat-related amygdala activation, and enhance functional coupling between the amygdala and medial prefrontal cortex and adjacent rostral cingulate cortex (mPFC/rACC) during threat processing in healthy adults.
Together, these findings suggest the cannabinoid system as a potential pharmacological target in the treatment of excess fear and anxiety. However, the effects of THC on corticolimbic functioning in response to threat have not be investigated in adults with trauma-related psychopathology.
To address this gap, the present study tests the effects of an acute low dose of THC on corticolimbic responses to threat in three groups of adults: (1) non-trauma-exposed healthy controls (HC; n = 25), (2) trauma-exposed adults without PTSD (TEC; n = 27), and (3) trauma-exposed adults with PTSD (n = 19).
Using a randomized, double-blind, placebo-controlled, between-subjects design, 71 participants were randomly assigned to receive either THC or placebo (PBO) and subsequently completed a well-established threat processing paradigm during functional magnetic resonance imaging.
In adults with PTSD, THC lowered threat-related amygdala reactivity, increased mPFC activation during threat, and increased mPFC-amygdala functional coupling.
These preliminary data suggest that THC modulates threat-related processing in trauma-exposed individuals with PTSD, which may prove advantageous as a pharmacological approach to treating stress- and trauma-related psychopathology.”
https://www.ncbi.nlm.nih.gov/pubmed/32162103
https://link.springer.com/article/10.1007%2Fs00213-020-05499-8

“Chronic pain is the most common reason reported for using medical cannabis.
The goal of this research was to determine if the two primary phytocannabinoids, THC and CBD, are effective treatments for persistent inflammatory pain.
These results suggest that THC may be more beneficial than CBD for reducing inflammatory pain, in that THC maintains its efficacy with short-term treatment in both sexes, and does not induce immune activation.
SIGNIFICANCE STATEMENT: CBDs and THCs pain-relieving effects are examined in male and female rats with persistent inflammatory pain to determine if individual phytocannabinoids could be a viable treatment for men and women with chronic inflammatory pain. Additionally, sex differences in the immune response to an adjuvant and to THC and CBD are characterized to provided preliminary insight into immune-related effects of cannabinoid-based therapy for pain.”
https://www.ncbi.nlm.nih.gov/pubmed/32179573
http://jpet.aspetjournals.org/content/early/2020/03/16/jpet.119.263319
 “Ratios of delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) impact metabolism and therapeutic effects of cannabis.
“Ratios of delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) impact metabolism and therapeutic effects of cannabis.
The medical and scientific communities have not drawn substantive conclusions nor thoroughly explored THC:CBD ratios for “best practice” treatment of different disease processes and their sequelae.
While there is evidence that cannabis provides medical benefits, research is lacking on standardization of medical cannabis use in modern medical practices.”
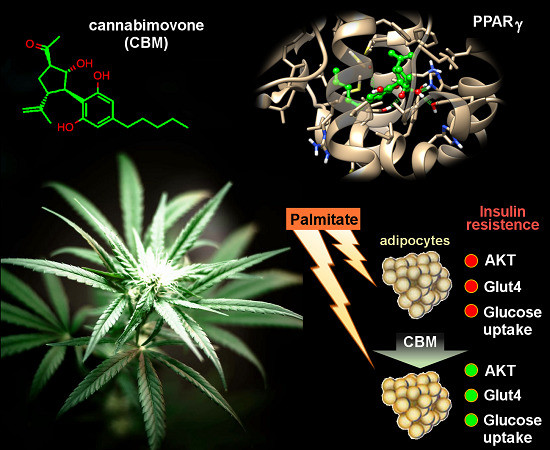
 “Phytocannabinoids (pCBs) are a large family of meroterpenoids isolated from the plant Cannabis sativa. Δ9-Tetrahydrocannabinol (THC) and cannabidiol (CBD) are the best investigated phytocannabinoids due to their relative abundance and interesting bioactivity profiles. In addition to various targets, THC and CBD are also well-known agonists of peroxisome proliferator-activated receptor gamma (PPARγ), a nuclear receptor involved in energy homeostasis and lipid metabolism. In the search of new pCBs potentially acting as PPARγ agonists, we identified cannabimovone (CBM), a structurally unique abeo-menthane pCB, as a novel PPARγ modulator via a combined computational and experimental approach. The ability of CBM to act as dual PPARγ/α agonist was also evaluated. Computational studies suggested a different binding mode toward the two isoforms, with the compound able to recapitulate the pattern of H-bonds of a canonical agonist only in the case of PPARγ. Luciferase assays confirmed the computational results, showing a selective activation of PPARγ by CBM in the low micromolar range. CBM promoted the expression of PPARγ target genes regulating the adipocyte differentiation and prevented palmitate-induced insulin signaling impairment. Altogether, these results candidate CBM as a novel bioactive compound potentially useful for the treatment of insulin resistance-related disorders.”
“Phytocannabinoids (pCBs) are a large family of meroterpenoids isolated from the plant Cannabis sativa. Δ9-Tetrahydrocannabinol (THC) and cannabidiol (CBD) are the best investigated phytocannabinoids due to their relative abundance and interesting bioactivity profiles. In addition to various targets, THC and CBD are also well-known agonists of peroxisome proliferator-activated receptor gamma (PPARγ), a nuclear receptor involved in energy homeostasis and lipid metabolism. In the search of new pCBs potentially acting as PPARγ agonists, we identified cannabimovone (CBM), a structurally unique abeo-menthane pCB, as a novel PPARγ modulator via a combined computational and experimental approach. The ability of CBM to act as dual PPARγ/α agonist was also evaluated. Computational studies suggested a different binding mode toward the two isoforms, with the compound able to recapitulate the pattern of H-bonds of a canonical agonist only in the case of PPARγ. Luciferase assays confirmed the computational results, showing a selective activation of PPARγ by CBM in the low micromolar range. CBM promoted the expression of PPARγ target genes regulating the adipocyte differentiation and prevented palmitate-induced insulin signaling impairment. Altogether, these results candidate CBM as a novel bioactive compound potentially useful for the treatment of insulin resistance-related disorders.”
https://www.ncbi.nlm.nih.gov/pubmed/32138197
https://www.mdpi.com/1420-3049/25/5/1119
 “Multiple sclerosis (MS) is a highly symptomatic disease, with a wide range of disabilities affecting many bodily functions, even in younger persons with a short disease history.
“Multiple sclerosis (MS) is a highly symptomatic disease, with a wide range of disabilities affecting many bodily functions, even in younger persons with a short disease history.
The availability of a cannabinoid oromucosal spray (Sativex) for the management of treatment-resistant MS spasticity has provided a new opportunity for many patients.
Our study aimed to assess the cost effectiveness of Sativex in Italian patients with treatment-resistant MS spasticity. The analysis was based on the real-world data of a large registry of Italian patients.
The use of Sativex could improve the quality of life of patients with a reasonable incremental cost resulting as a cost-effective option for patients with MS-resistant spasticity. These results could help clinicians and decision makers to develop improved management strategies for spasticity in patients with MS, optimizing the use of available resources.”
https://www.ncbi.nlm.nih.gov/pubmed/32130684
https://link.springer.com/article/10.1007%2Fs40261-020-00895-6

“Breast cancer (BC) is the second most prevalent cancer worldwide.
Estrogen receptor beta (ERβ) is an essential protein of breast cells to suppress estrogen induced uncontrolled proliferation. Thus small molecules that can modulate and enhance ERβ expression would be an effective agent to suppress BC development.
Studies showed that cannabinoid (CB), specifically Delta-9-tetrahydrocannabinol (Del9THC), can increase the expression of ERβ and inhibits BC cell proliferation.
In this study, less psychoactive and structurally similar analogues of Del9THC were chosen as drug candidates and ERβ was targeted as a therapeutic receptor. Delta-8-tetrahydrocannabinol (Del8THC) and Delta-4-Isotetrahydrocannabinol (Del4isoTHC) were the drug candidates selected on the basis of literature reports, Absorption, Distribution, Metabolism, Excretion and Toxicity (ADMET) properties, medicinal chemistry profile and physicochemical features.
Molecular docking simulations were carried out to determine ligand receptor interactions and binding affinity based on free binding energy. To get a better drug, the structural modification was done on Del8THC and generated a new CB analogue called Cannabinoid A.
Finally, molecular interaction analysis revealed that two CBs and one of their analogue interact with the active site residues of ERβ. Therefore, this study revealed a new way to discover novel drug(s) for BC patients.”
https://www.ncbi.nlm.nih.gov/pubmed/32116130
https://www.tandfonline.com/doi/abs/10.1080/07391102.2020.1737233?journalCode=tbsd20
 “Breast cancer (BC) is the most common cancer in women worldwide. Approximately 70-80% of BCs express estrogen receptors (ER), which predict the response to endocrine therapy (ET), and are therefore hormone receptor-positive (HR+).
“Breast cancer (BC) is the most common cancer in women worldwide. Approximately 70-80% of BCs express estrogen receptors (ER), which predict the response to endocrine therapy (ET), and are therefore hormone receptor-positive (HR+).
Endogenous cannabinoids together with cannabinoid receptor 1 and 2 (CB1, CB2) constitute the basis of the endocannabinoid system.
Interactions of cannabinoids with hypothalamic-pituitary-gonadal axis hormones are well documented, and two studies found a positive correlation between peak plasma endogenous cannabinoid anandamide with peak plasma 17β-estradiol, luteinizing hormone and follicle-stimulating hormone levels at ovulation in healthy premenopausal women. Do cannabinoids have an effect on HR+ BC? In this paper we review known and possible interactions between cannabinoids and specific HR+ BC treatments.
In preclinical studies, CB1 and CB2 agonists (i.e., anandamide, THC) have been shown to inhibit the proliferation of ER positive BC cell lines.
There is less evidence for antitumor cannabinoid action in HR+ BC in animal models and there are no clinical trials exploring the effects of cannabinoids on HR+ BC treatment outcomes. Two studies have shown that tamoxifen and several other selective estrogen receptor modulators (SERM) can act as inverse agonists on CB1 and CB2, an interaction with possible clinical consequences. In addition, cannabinoid action could interact with other commonly used endocrine and targeted therapies used in the treatment of HR+ BC.”
https://www.ncbi.nlm.nih.gov/pubmed/32106399
https://www.mdpi.com/2072-6694/12/3/525
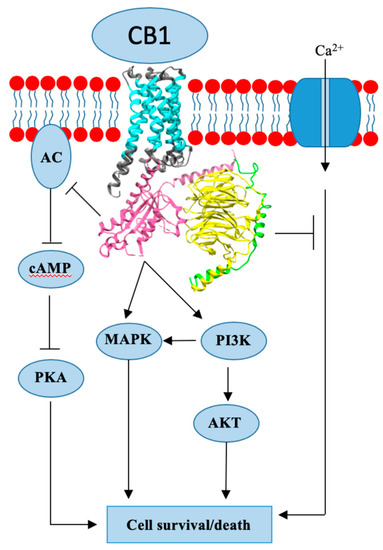
 “The 20% prevalence of chronic pain in the general population is a major health concern given the often profound associated impairment of daily activities, employment status, and health-related quality of life in sufferers. Resource utilization associated with chronic pain represents an enormous burden for healthcare systems. Although analgesia based on the World Health Organization’s pain ladder continues to be the mainstay of chronic pain management, aside from chronic cancer pain or end-of-life care, prolonged use of non-steroidal anti-inflammatory drugs or opioids to manage chronic pain is rarely sustainable.
“The 20% prevalence of chronic pain in the general population is a major health concern given the often profound associated impairment of daily activities, employment status, and health-related quality of life in sufferers. Resource utilization associated with chronic pain represents an enormous burden for healthcare systems. Although analgesia based on the World Health Organization’s pain ladder continues to be the mainstay of chronic pain management, aside from chronic cancer pain or end-of-life care, prolonged use of non-steroidal anti-inflammatory drugs or opioids to manage chronic pain is rarely sustainable.
As the endocannabinoid system is known to control pain at peripheral, spinal, and supraspinal levels, interest in medical use of cannabis is growing.
A proprietary blend of cannabis plant extracts containing delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) as the principal cannabinoids is formulated as an oromucosal spray (USAN name: nabiximols) and standardized to ensure quality, consistency and stability. This review examines evidence for THC:CBD oromucosal spray (nabiximols) in the management of chronic pain conditions.
Cumulative evidence from clinical trials and an exploratory analysis of the German Pain e-Registry suggests that add-on THC:CBD oromucosal spray (nabiximols) may have a role in managing chronic neuropathic pain, although further precise clinical trials are required to draw definitive conclusions.”
https://www.ncbi.nlm.nih.gov/pubmed/32104061
“Smoked Cannabis Proven Effective In Treating Neuropathic Pain.” https://www.sciencedaily.com/releases/2007/10/071024141745.htm
“Marijuana Relieves Chronic Pain, Research Shows” https://www.webmd.com/pain-management/news/20100830/marijuana-relieves-chronic-pain-research-show#1
 “Burning mouth syndrome (BMS) is a neuropathic pain disorder associated with a burning sensation on oral mucosal surfaces with frequently reported xerostomia, dysgeusia and tingling or paraesthetic sensations. However, patients present no clinically evident causative lesions. The poor classification of the disorder has resulted in a diagnostic challenge, particularly for the clinician/dentist evaluating these individuals. Major research developments have been made in the BMS field in recent years to address this concern, principally in terms of the pathophysiological mechanisms underlying the disorder, in addition to therapeutic advancements. For the purpose of this review, an update on the pathophysiological mechanisms will be discussed from a neuropathic, immunological, hormonal and psychological perspective. This review will also focus on the many therapeutic strategies that have been explored for BMS, including antidepressants/antipsychotics, nonsteroidal anti-inflammatories, hormone replacement therapies, phytotherapeutic compounds and non-pharmacological interventions, overall highlighting the lack of controlled clinical studies to support the effectiveness of such therapeutic avenues. Particular focus is given to the cannabinoid system, and the potential of cannabis-based therapeutics in managing BMS patients.”
“Burning mouth syndrome (BMS) is a neuropathic pain disorder associated with a burning sensation on oral mucosal surfaces with frequently reported xerostomia, dysgeusia and tingling or paraesthetic sensations. However, patients present no clinically evident causative lesions. The poor classification of the disorder has resulted in a diagnostic challenge, particularly for the clinician/dentist evaluating these individuals. Major research developments have been made in the BMS field in recent years to address this concern, principally in terms of the pathophysiological mechanisms underlying the disorder, in addition to therapeutic advancements. For the purpose of this review, an update on the pathophysiological mechanisms will be discussed from a neuropathic, immunological, hormonal and psychological perspective. This review will also focus on the many therapeutic strategies that have been explored for BMS, including antidepressants/antipsychotics, nonsteroidal anti-inflammatories, hormone replacement therapies, phytotherapeutic compounds and non-pharmacological interventions, overall highlighting the lack of controlled clinical studies to support the effectiveness of such therapeutic avenues. Particular focus is given to the cannabinoid system, and the potential of cannabis-based therapeutics in managing BMS patients.”