“Background: Cannabis for cancer is very topical and, given the use of illicit cannabis preparations used in this vulnerable population, research investigating standardised, quality-assured medicinal cannabis is critical to inform clinicians and assist patient safety.
“Background: Cannabis for cancer is very topical and, given the use of illicit cannabis preparations used in this vulnerable population, research investigating standardised, quality-assured medicinal cannabis is critical to inform clinicians and assist patient safety.
Methods: A randomized trial involving adult patients diagnosed with a high-grade glioma, no history of substance abuse, liver or kidney damage or myocardial infarction were eligible for inclusion in a tolerability study on two different ratios of medicinal cannabis. Baseline screening of brain morphology, blood pathology, functional status, and cognition was conducted. A retrospective control group was used for comparison for secondary outcomes.
Results: Participants (n=88) were on average 53.3 years old. A paired t-test assessed the Functional Assessment of Cancer Therapy for Brain Cancer (FACT-Br) between groups from baseline to week 12 found that the 1:1 ratio favoured both physical (p=0.025) and functional (p=0.014) capacity and improved sleep (p=0.009). Analysis of changes from baseline to week 12 also found 11% of 61 participants had a reduction in disease, 34% were stable, 16% had slight enhancement, and 10% had progressive disease. No serious adverse events occurred. Side effects included dry mouth, tiredness at night, dizziness, drowsiness.
Conclusion: This study demonstrated that a single nightly dose of THC-containing medicinal cannabis was safe, had no serious adverse effects and was well tolerated in patients. Medicinal cannabis significantly improved sleep, functional wellbeing, and quality of life.”
https://pubmed.ncbi.nlm.nih.gov/34094937/
“From this study we have shown that a single nightly dose of THC-containing cannabis was well tolerated in patients in both groups with high-grade gliomas and significantly improved sleep, functional wellbeing and contentment with QoL in a sample of patients compared to baseline. From this trial, the 1:1 ratio has been identified as the preferred combination the moving forward to further trials. This study significantly informs MC product choice for ongoing studies into cannabis being a potential adjunct treatment option for this patient population.”
https://www.frontiersin.org/articles/10.3389/fonc.2021.649555/full

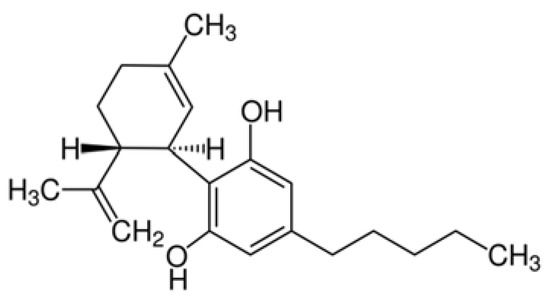
 “The cannabinoid, cannabidiol (CBD), is part of the plant’s natural defense system that when given to animals has many useful medicinal properties, including activity against cancer cells, modulation of the immune system, and efficacy in epilepsy.
“The cannabinoid, cannabidiol (CBD), is part of the plant’s natural defense system that when given to animals has many useful medicinal properties, including activity against cancer cells, modulation of the immune system, and efficacy in epilepsy.  “Cannabis
“Cannabis “Foodborne protein hydrolysates exhibit biological activity that may be therapeutic in a number of human disease settings. Hemp peptides (HP) generated by controlled hydrolysis of hemp proteins have a number of health benefits and are of pharmaceutical value. In the present study, we produce small molecular weight HP from hemp seed and investigate its anticancer properties in Hep3B human liver cancer cells. We demonstrate that HP treatment increased apoptosis, reduced cell viability, and reduced cell migration in Hep3B human liver cancer cells without affecting the normal liver cell line L02. We correlate these phenotypes with increased cellular ROS levels, upregulation of cleaved caspase 3 and Bad, and downregulation of antiapoptotic Bcl-2. HP treatment led to increased Akt and GSK-3β phosphorylation, with subsequent downregulation of β-catenin, suggesting β-catenin signaling modulation as a critical mechanism by which HP exhibits anticancer properties. Our findings suggest HP are of potential therapeutic interest for liver cancer treatment.”
“Foodborne protein hydrolysates exhibit biological activity that may be therapeutic in a number of human disease settings. Hemp peptides (HP) generated by controlled hydrolysis of hemp proteins have a number of health benefits and are of pharmaceutical value. In the present study, we produce small molecular weight HP from hemp seed and investigate its anticancer properties in Hep3B human liver cancer cells. We demonstrate that HP treatment increased apoptosis, reduced cell viability, and reduced cell migration in Hep3B human liver cancer cells without affecting the normal liver cell line L02. We correlate these phenotypes with increased cellular ROS levels, upregulation of cleaved caspase 3 and Bad, and downregulation of antiapoptotic Bcl-2. HP treatment led to increased Akt and GSK-3β phosphorylation, with subsequent downregulation of β-catenin, suggesting β-catenin signaling modulation as a critical mechanism by which HP exhibits anticancer properties. Our findings suggest HP are of potential therapeutic interest for liver cancer treatment.” “Cannabinoids are a family of heterogeneous compounds that mostly interact with receptors eliciting several physiological effects both in the central and peripheral nervous systems and in peripheral organs. They exert anticancer action by modulating signaling pathways involved in cancer progression; furthermore, the effects induced by their use depend on both the type of tumor and their action on the components of the endocannabinoid system. This review will explore the mechanism of action of the cannabinoids in signaling pathways involved in cancer proliferation, neovascularisation, migration, invasion, metastasis, and tumor angiogenesis.”
“Cannabinoids are a family of heterogeneous compounds that mostly interact with receptors eliciting several physiological effects both in the central and peripheral nervous systems and in peripheral organs. They exert anticancer action by modulating signaling pathways involved in cancer progression; furthermore, the effects induced by their use depend on both the type of tumor and their action on the components of the endocannabinoid system. This review will explore the mechanism of action of the cannabinoids in signaling pathways involved in cancer proliferation, neovascularisation, migration, invasion, metastasis, and tumor angiogenesis.”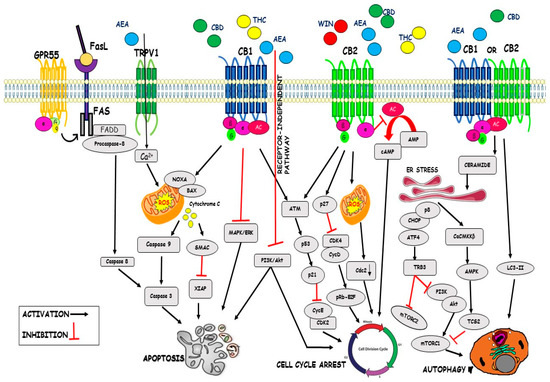
 “Agents targeting the endocannabinoid system (ECS) have gained attention as potential cancer treatments. Given recent evidence that cannabinoid receptor 2 (CB2R) regulates lymphocyte development and inflammation, we performed studies on CB2R in the immune response against melanoma. Analysis of The Cancer Genome Atlas (TCGA) data revealed a strong positive correlation between CB2R expression and survival, as well as B cell infiltration in human melanoma. In a murine melanoma model, CB2R expression reduced the growth of melanoma as well as the B cell frequencies in the tumor microenvironment (TME), compared to CB2R-deficient mice. In depth analysis of tumor-infiltrating B cells using single-cell RNA sequencing suggested a less differentiated phenotype in tumors from
“Agents targeting the endocannabinoid system (ECS) have gained attention as potential cancer treatments. Given recent evidence that cannabinoid receptor 2 (CB2R) regulates lymphocyte development and inflammation, we performed studies on CB2R in the immune response against melanoma. Analysis of The Cancer Genome Atlas (TCGA) data revealed a strong positive correlation between CB2R expression and survival, as well as B cell infiltration in human melanoma. In a murine melanoma model, CB2R expression reduced the growth of melanoma as well as the B cell frequencies in the tumor microenvironment (TME), compared to CB2R-deficient mice. In depth analysis of tumor-infiltrating B cells using single-cell RNA sequencing suggested a less differentiated phenotype in tumors from 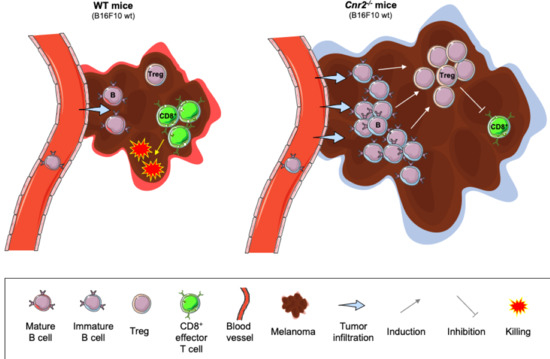
 “Colorectal cancer (CRC) is between the top three occurring cancers worldwide. The anticancer effects of Cannabinoid receptor 2 (CB
“Colorectal cancer (CRC) is between the top three occurring cancers worldwide. The anticancer effects of Cannabinoid receptor 2 (CB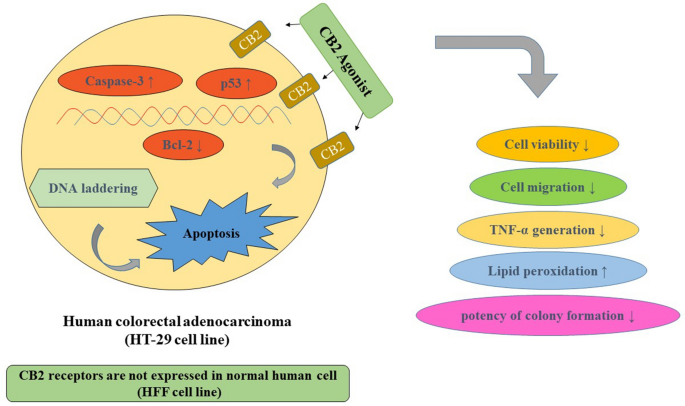
 “In recent years, evidence has accumulated that cannabinoids-especially the non-psychoactive compound, cannabidiol (CBD)-possess promising medical and pharmacological activities that might qualify them as potential anti-tumor drugs. This review is based on multiple studies summarizing different mechanisms for how CBD can target tumor cells including cannabinoid receptors or other constituents of the endocannabinoid system, and their complex activation of biological systems that results in the inhibition of tumor growth. CBD also participates in anti-inflammatory activities which are related to tumor progression, as demonstrated in preclinical models. Although the numbers of clinical trials and tested tumor entities are limited, there is clear evidence that CBD has anti-tumor efficacy and is well tolerated in human cancer patients. In summary, it appears that CBD has potential as a neoadjuvant and/or adjuvant drug in therapy for cancer.”
“In recent years, evidence has accumulated that cannabinoids-especially the non-psychoactive compound, cannabidiol (CBD)-possess promising medical and pharmacological activities that might qualify them as potential anti-tumor drugs. This review is based on multiple studies summarizing different mechanisms for how CBD can target tumor cells including cannabinoid receptors or other constituents of the endocannabinoid system, and their complex activation of biological systems that results in the inhibition of tumor growth. CBD also participates in anti-inflammatory activities which are related to tumor progression, as demonstrated in preclinical models. Although the numbers of clinical trials and tested tumor entities are limited, there is clear evidence that CBD has anti-tumor efficacy and is well tolerated in human cancer patients. In summary, it appears that CBD has potential as a neoadjuvant and/or adjuvant drug in therapy for cancer.”