 “Background: Cannabis for cancer is very topical and, given the use of illicit cannabis preparations used in this vulnerable population, research investigating standardised, quality-assured medicinal cannabis is critical to inform clinicians and assist patient safety.
“Background: Cannabis for cancer is very topical and, given the use of illicit cannabis preparations used in this vulnerable population, research investigating standardised, quality-assured medicinal cannabis is critical to inform clinicians and assist patient safety.
Methods: A randomized trial involving adult patients diagnosed with a high-grade glioma, no history of substance abuse, liver or kidney damage or myocardial infarction were eligible for inclusion in a tolerability study on two different ratios of medicinal cannabis. Baseline screening of brain morphology, blood pathology, functional status, and cognition was conducted. A retrospective control group was used for comparison for secondary outcomes.
Results: Participants (n=88) were on average 53.3 years old. A paired t-test assessed the Functional Assessment of Cancer Therapy for Brain Cancer (FACT-Br) between groups from baseline to week 12 found that the 1:1 ratio favoured both physical (p=0.025) and functional (p=0.014) capacity and improved sleep (p=0.009). Analysis of changes from baseline to week 12 also found 11% of 61 participants had a reduction in disease, 34% were stable, 16% had slight enhancement, and 10% had progressive disease. No serious adverse events occurred. Side effects included dry mouth, tiredness at night, dizziness, drowsiness.
Conclusion: This study demonstrated that a single nightly dose of THC-containing medicinal cannabis was safe, had no serious adverse effects and was well tolerated in patients. Medicinal cannabis significantly improved sleep, functional wellbeing, and quality of life.”
https://pubmed.ncbi.nlm.nih.gov/34094937/
“From this study we have shown that a single nightly dose of THC-containing cannabis was well tolerated in patients in both groups with high-grade gliomas and significantly improved sleep, functional wellbeing and contentment with QoL in a sample of patients compared to baseline. From this trial, the 1:1 ratio has been identified as the preferred combination the moving forward to further trials. This study significantly informs MC product choice for ongoing studies into cannabis being a potential adjunct treatment option for this patient population.”
https://www.frontiersin.org/articles/10.3389/fonc.2021.649555/full

 “Glioblastoma multiforme (GBM) is the most lethal subtype of glioma.
“Glioblastoma multiforme (GBM) is the most lethal subtype of glioma. 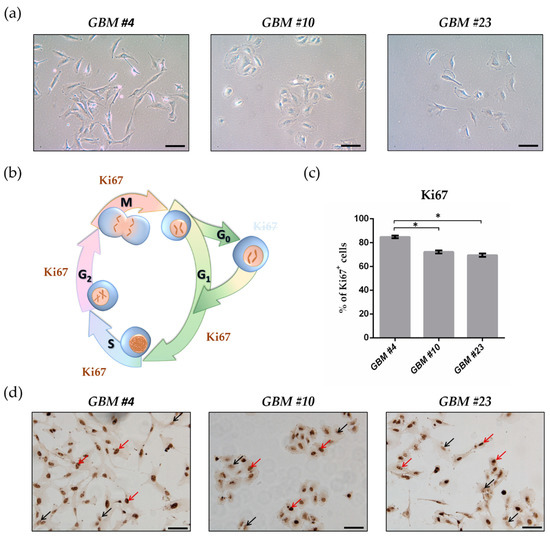

 “Cannabidiol (CBD) has been shown to slow cancer cell growth and is toxic to human glioblastoma cell lines. Thus, CBD could be an effective therapeutic for glioblastoma.
“Cannabidiol (CBD) has been shown to slow cancer cell growth and is toxic to human glioblastoma cell lines. Thus, CBD could be an effective therapeutic for glioblastoma.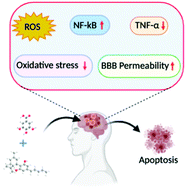
 “Preclinical data suggest some cannabinoids may exert antitumour effects against glioblastoma (GBM). Safety and preliminary efficacy of nabiximols oromucosal cannabinoid spray plus dose-intense temozolomide (DIT) was evaluated in patients with first recurrence of GBM.
“Preclinical data suggest some cannabinoids may exert antitumour effects against glioblastoma (GBM). Safety and preliminary efficacy of nabiximols oromucosal cannabinoid spray plus dose-intense temozolomide (DIT) was evaluated in patients with first recurrence of GBM.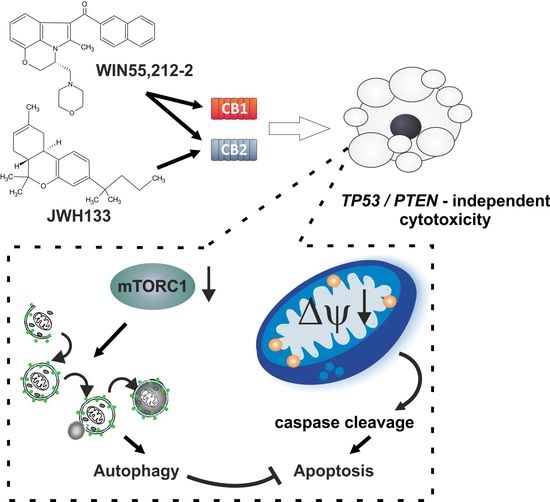
 “Glioblastoma is the most aggressive cancer among primary brain tumours. As with other cancers, the incidence of glioblastoma is increasing; despite modern therapies, the overall mean survival of patients post-diagnosis averages around 16 months, a figure that has not changed in many years. Cannabigerol (CBG) has only recently been reported to prevent the progression of certain carcinomas and has not yet been studied in glioblastoma. Here, we have compared the cytotoxic, apoptotic, and anti-invasive effects of the purified natural cannabinoid CBG together with CBD and THC on established differentiated glioblastoma tumour cells and glioblastoma stem cells. CBG and THC reduced the viability of both types of cells to a similar extent, whereas combining CBD with CBG was more efficient than with THC. CBD and CBG, both alone and in combination, induced caspase-dependent cell apoptosis, and there was no additive THC effect. Of note, CBG inhibited glioblastoma invasion in a similar manner to CBD and the chemotherapeutic temozolomide. We have demonstrated that THC has little added value in combined-cannabinoid glioblastoma treatment, suggesting that this psychotropic cannabinoid should be replaced with CBG in future clinical studies of glioblastoma therapy.”
“Glioblastoma is the most aggressive cancer among primary brain tumours. As with other cancers, the incidence of glioblastoma is increasing; despite modern therapies, the overall mean survival of patients post-diagnosis averages around 16 months, a figure that has not changed in many years. Cannabigerol (CBG) has only recently been reported to prevent the progression of certain carcinomas and has not yet been studied in glioblastoma. Here, we have compared the cytotoxic, apoptotic, and anti-invasive effects of the purified natural cannabinoid CBG together with CBD and THC on established differentiated glioblastoma tumour cells and glioblastoma stem cells. CBG and THC reduced the viability of both types of cells to a similar extent, whereas combining CBD with CBG was more efficient than with THC. CBD and CBG, both alone and in combination, induced caspase-dependent cell apoptosis, and there was no additive THC effect. Of note, CBG inhibited glioblastoma invasion in a similar manner to CBD and the chemotherapeutic temozolomide. We have demonstrated that THC has little added value in combined-cannabinoid glioblastoma treatment, suggesting that this psychotropic cannabinoid should be replaced with CBG in future clinical studies of glioblastoma therapy.”