 “There is increasing interest in the use of purified cannabidiol (CBD) as a treatment for a wide range of conditions due to its reported anti-inflammatory, anxiolytic, antiemetic and anticonvulsant properties.
“There is increasing interest in the use of purified cannabidiol (CBD) as a treatment for a wide range of conditions due to its reported anti-inflammatory, anxiolytic, antiemetic and anticonvulsant properties.
OBJECTIVE:
The objective of this study was to assess the safety, tolerability and pharmacokinetics of a single ascending dose of a new lipid-based oral formulation of CBD in healthy volunteers after a high-fat meal.
RESULTS:
CBD was well tolerated in the healthy volunteers (mean age: 24.0 years) treated with a single oral dose of CBD. There were no safety concerns with increasing the dose and the safety profiles of the CBD-treated and placebo-treated subjects were similar. The most frequently reported treatment emergent adverse events (TEAEs) were headache (17%) and diarrhoea (8%). There were no reported serious adverse events (SAEs) and no clinical laboratory findings, vital signs, ECGs or physical examination findings that were reported as TEAEs or were of clinical significance during the study. After a high-fat meal, CBD was detected in plasma samples at 15 min postdose; the median time to maximum plasma concentration (Tmax) was 4 h across all three CBD dose cohorts. The CBD plasma exposure [maximum observed plasma concentration (Cmax) and the area under the concentration-time curve (AUC)] increased in a dose-proportional manner and declined to levels approaching the lower level of quantification by day 8. The terminal elimination half-life was approximately 70 h, suggesting that 2-3 weeks are needed to fully eliminate CBD.
CONCLUSIONS:
This new CBD formulation demonstrated a favourable safety and tolerability profile in healthy volunteers that was consistent with the profiles reported for other purified CBD products. No severe or serious AEs were observed in this study and there were no safety concerns.”
https://www.ncbi.nlm.nih.gov/pubmed/32409982
“Cannabidiol (CBD) is a major nonpsychoactive cannabinoid derived from the Cannabis plant that has attracted significant interest due to its anti-inflammatory, anxiolytic, antiemetic and anticonvulsant properties. The findings of this study contribute to the evolving knowledge of cannabidiol pharmacokinetics and indicate that this new oral lipid-based formulation of cannabidiol is generally safe and well tolerated at all doses studied. No severe or serious AEs were observed and there were no safety concerns.”
https://link.springer.com/article/10.1007%2Fs13318-020-00624-6

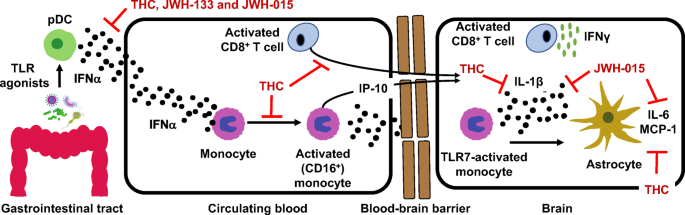
 “Opioid prescriptions and abuse remain a significant national concern.
“Opioid prescriptions and abuse remain a significant national concern. “Modern day research, in an attempt to determine the potential therapeutic and adverse effects of illicit substances, is a growing field, but one that faces many regulatory challenges. Due to the potential abuse of illicit substances such as
“Modern day research, in an attempt to determine the potential therapeutic and adverse effects of illicit substances, is a growing field, but one that faces many regulatory challenges. Due to the potential abuse of illicit substances such as 

 “The intraperitoneal administration of chemotherapeutics has emerged as a potential route in ovarian cancer treatment. Nanoparticles as carriers for these agents could be interesting by increasing the retention of chemotherapeutics within the peritoneal cavity. Moreover, nanoparticles could be internalised by cancer cells and let the drug release near the biological target, which could increase the anticancer efficacy.
“The intraperitoneal administration of chemotherapeutics has emerged as a potential route in ovarian cancer treatment. Nanoparticles as carriers for these agents could be interesting by increasing the retention of chemotherapeutics within the peritoneal cavity. Moreover, nanoparticles could be internalised by cancer cells and let the drug release near the biological target, which could increase the anticancer efficacy.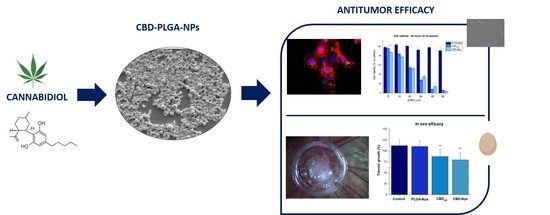
.jpg) “Despite limited data demonstrating pronounced negative effects of prenatal cannabis exposure, popular opinion and public policies still reflect the belief that cannabis is fetotoxic.
“Despite limited data demonstrating pronounced negative effects of prenatal cannabis exposure, popular opinion and public policies still reflect the belief that cannabis is fetotoxic. “The antioxidant and CB2 receptor agonist properties of Δ9-tetrahydrocannabivarin (Δ9-THCV) afforded neuroprotection in experimental Parkinson’s disease (PD), whereas its CB1 receptor antagonist profile at doses lower than 5 mg/kg caused anti-hypokinetic effects.
“The antioxidant and CB2 receptor agonist properties of Δ9-tetrahydrocannabivarin (Δ9-THCV) afforded neuroprotection in experimental Parkinson’s disease (PD), whereas its CB1 receptor antagonist profile at doses lower than 5 mg/kg caused anti-hypokinetic effects. “Lenabasum is an oral synthetic
“Lenabasum is an oral synthetic