 “Neurological disorders such as neurodegenerative diseases or traumatic brain injury are associated with cognitive, motor and behavioural changes that influence the quality of life of the patients. Although different therapeutic strategies have been developed and tried until now to decrease the neurological decline, no treatment has been found to cure these pathologies.
“Neurological disorders such as neurodegenerative diseases or traumatic brain injury are associated with cognitive, motor and behavioural changes that influence the quality of life of the patients. Although different therapeutic strategies have been developed and tried until now to decrease the neurological decline, no treatment has been found to cure these pathologies.
In the last decades, the implication of the endocannabinoid system in the neurological function has been extensively studied, and the cannabinoids have been tried as a new promising potential treatment. In this study, we aimed to overview the recent available literature regarding in vivo potential of natural and synthetic cannabinoids with underlying mechanisms of action for protecting against cognitive decline and motor impairments.
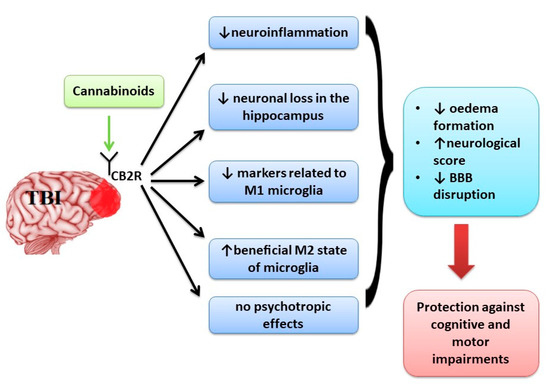
The results of studies on animal models showed that cannabinoids in traumatic brain injury increase neurobehavioral function, working memory performance, and decrease the neurological deficit and ameliorate motor deficit through down-regulation of pro-inflammatory markers, oedema formation and blood-brain barrier permeability, preventing neuronal cell loss and up-regulating the levels of adherence junction proteins.
In neurodegenerative diseases, the cannabinoids showed beneficial effects in decreasing the motor disability and disease progression by a complex mechanism targeting more signalling pathways further than classical receptors of the endocannabinoid system. In light of these results, the use of cannabinoids could be beneficial in traumatic brain injuries and multiple sclerosis treatment, especially in those patients who display resistance to conventional treatment.”
https://pubmed.ncbi.nlm.nih.gov/32726998/
https://www.mdpi.com/2077-0383/9/8/2395

 “Objectives: To investigate the action of cannabinoids on spasticity and pain in secondary progressive multiple sclerosis, by means of neurophysiological indexes.
“Objectives: To investigate the action of cannabinoids on spasticity and pain in secondary progressive multiple sclerosis, by means of neurophysiological indexes. “Painful tonic spasm (PTS) is a common yet debilitating symptom in patients with neuromyelitis optica spectrum disorder (NMOSD), especially those with longitudinally extensive transverse myelitis. Although carbamazepine is an effective treatment, it poses the risk of severe adverse reactions, such as Steven-Johnson syndrome (SJS).
“Painful tonic spasm (PTS) is a common yet debilitating symptom in patients with neuromyelitis optica spectrum disorder (NMOSD), especially those with longitudinally extensive transverse myelitis. Although carbamazepine is an effective treatment, it poses the risk of severe adverse reactions, such as Steven-Johnson syndrome (SJS). “Cannabidiol (
“Cannabidiol ( “Moderate to severe spasticity is commonly reported in Multiple Sclerosis (MS) and its management is still a challenge. Cannabinoids were recently suggested as add-on therapy for the treatment of spasticity and chronic pain in MS but there is no conclusive scientific evidence on their safety, especially on cognition and over long periods.
“Moderate to severe spasticity is commonly reported in Multiple Sclerosis (MS) and its management is still a challenge. Cannabinoids were recently suggested as add-on therapy for the treatment of spasticity and chronic pain in MS but there is no conclusive scientific evidence on their safety, especially on cognition and over long periods. “Multiple sclerosis (MS) is an autoimmune demyelinating disease of the central nervous system, affecting ambulation even in people with only mild neurological signs. Patients with MS frequently experience spasticity, which contributes significantly to impair their motor functions, including ambulation, owing to muscle stiffness, spasms, and pain.
“Multiple sclerosis (MS) is an autoimmune demyelinating disease of the central nervous system, affecting ambulation even in people with only mild neurological signs. Patients with MS frequently experience spasticity, which contributes significantly to impair their motor functions, including ambulation, owing to muscle stiffness, spasms, and pain.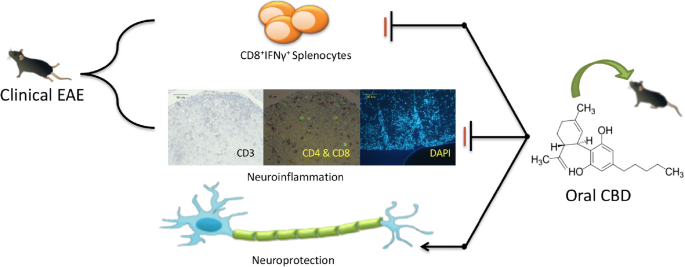
 “The pathophysiological relevance of the endocannabinoid system has been widely demonstrated in a variety of diseases including cancer, neurological disorders, and metabolic issues. Therefore, targeting the receptors and the endogenous machinery involved in this system can provide a successful therapeutic outcome.
“The pathophysiological relevance of the endocannabinoid system has been widely demonstrated in a variety of diseases including cancer, neurological disorders, and metabolic issues. Therefore, targeting the receptors and the endogenous machinery involved in this system can provide a successful therapeutic outcome. “The approval of 9-δ-tetrahydocannabinol (THC)+
“The approval of 9-δ-tetrahydocannabinol (THC)+