 “Depression is a widespread psychological disorder that affects up to 20% of the world’s population. Traditional Chinese medicine (TCM), with its unique curative effect in depression treatment, is gaining increasing attention as the discovery of novel antidepressant drug has become the pursuit of pharmaceutical. This article summarizes the work done on the natural products from TCM that have been reported to conceive antidepressant effects in the past two decades, which can be classified according to various mechanisms including increasing synaptic concentrations of monoamines, alleviating the hypothalamic-pituitary-adrenal (HPA) axis dysfunctions, lightening the impairment of neuroplasticity, fighting towards immune and inflammatory dysregulation. The antidepressant active ingredients identified can be generally divided into saponins, flavonoids, alkaloids, polysaccharides and others. Albiflorin, Baicalein, Berberine chloride, beta-Asarone, cannabidiol, Curcumin, Daidzein, Echinocystic acid (EA), Emodin, Ferulic acid, Gastrodin, Genistein, Ginsenoside Rb1, Ginsenoside Rg1, Ginsenoside Rg3, Hederagenin, Hesperidin, Honokiol, Hyperoside, Icariin, Isoliquiritin, Kaempferol, Liquiritin, L-theanine, Magnolol, Paeoniflorin, Piperine, Proanthocyanidin, Puerarin, Quercetin, Resveratrol (trans), Rosmarinic acid, Saikosaponin A, Senegenin, Tetrahydroxystilbene glucoside and Vanillic acid are Specified in this review. Simultaneously, chemical structures of the active ingredients with antidepressant activities are listed and their sources, models, efficacy and mechanisms are described. Chinese compound prescription and extracts that exert antidepressant effects are also introduced, which may serve as a source of inspiration for further development. In the view of present study, the antidepressant effect of certain TCMs are affirmative and encouraging. However, there are a lot of work needs to be done to evaluate the exact therapeutic effects and mechanisms of those active ingredients, specifically, to establish a unified standard for diagnosis and evaluation of curative effect.”
“Depression is a widespread psychological disorder that affects up to 20% of the world’s population. Traditional Chinese medicine (TCM), with its unique curative effect in depression treatment, is gaining increasing attention as the discovery of novel antidepressant drug has become the pursuit of pharmaceutical. This article summarizes the work done on the natural products from TCM that have been reported to conceive antidepressant effects in the past two decades, which can be classified according to various mechanisms including increasing synaptic concentrations of monoamines, alleviating the hypothalamic-pituitary-adrenal (HPA) axis dysfunctions, lightening the impairment of neuroplasticity, fighting towards immune and inflammatory dysregulation. The antidepressant active ingredients identified can be generally divided into saponins, flavonoids, alkaloids, polysaccharides and others. Albiflorin, Baicalein, Berberine chloride, beta-Asarone, cannabidiol, Curcumin, Daidzein, Echinocystic acid (EA), Emodin, Ferulic acid, Gastrodin, Genistein, Ginsenoside Rb1, Ginsenoside Rg1, Ginsenoside Rg3, Hederagenin, Hesperidin, Honokiol, Hyperoside, Icariin, Isoliquiritin, Kaempferol, Liquiritin, L-theanine, Magnolol, Paeoniflorin, Piperine, Proanthocyanidin, Puerarin, Quercetin, Resveratrol (trans), Rosmarinic acid, Saikosaponin A, Senegenin, Tetrahydroxystilbene glucoside and Vanillic acid are Specified in this review. Simultaneously, chemical structures of the active ingredients with antidepressant activities are listed and their sources, models, efficacy and mechanisms are described. Chinese compound prescription and extracts that exert antidepressant effects are also introduced, which may serve as a source of inspiration for further development. In the view of present study, the antidepressant effect of certain TCMs are affirmative and encouraging. However, there are a lot of work needs to be done to evaluate the exact therapeutic effects and mechanisms of those active ingredients, specifically, to establish a unified standard for diagnosis and evaluation of curative effect.”
https://www.ncbi.nlm.nih.gov/pubmed/31706012
https://www.sciencedirect.com/science/article/abs/pii/S1043661819322601?via%3Dihub
 “Accumulating evidence implicates the endocannabinoid system in the pathophysiology of psychosis.
“Accumulating evidence implicates the endocannabinoid system in the pathophysiology of psychosis.
 “Medicinal
“Medicinal 
 “Depression is a widespread psychological disorder that affects up to 20% of the world’s population. Traditional Chinese medicine (TCM), with its unique curative effect in depression treatment, is gaining increasing attention as the discovery of novel antidepressant drug has become the pursuit of pharmaceutical. This article summarizes the work done on the natural products from TCM that have been reported to conceive antidepressant effects in the past two decades, which can be classified according to various mechanisms including increasing synaptic concentrations of monoamines, alleviating the hypothalamic-pituitary-adrenal (HPA) axis dysfunctions, lightening the impairment of neuroplasticity, fighting towards immune and inflammatory dysregulation. The antidepressant active ingredients identified can be generally divided into saponins, flavonoids, alkaloids, polysaccharides and others. Albiflorin, Baicalein, Berberine chloride, beta-Asarone, cannabidiol, Curcumin, Daidzein, Echinocystic acid (EA), Emodin, Ferulic acid, Gastrodin, Genistein, Ginsenoside Rb1, Ginsenoside Rg1, Ginsenoside Rg3, Hederagenin, Hesperidin, Honokiol, Hyperoside, Icariin, Isoliquiritin, Kaempferol, Liquiritin, L-theanine, Magnolol, Paeoniflorin, Piperine, Proanthocyanidin, Puerarin, Quercetin, Resveratrol (trans), Rosmarinic acid, Saikosaponin A, Senegenin, Tetrahydroxystilbene glucoside and Vanillic acid are Specified in this review. Simultaneously, chemical structures of the active ingredients with antidepressant activities are listed and their sources, models, efficacy and mechanisms are described. Chinese compound prescription and extracts that exert antidepressant effects are also introduced, which may serve as a source of inspiration for further development. In the view of present study, the antidepressant effect of certain TCMs are affirmative and encouraging. However, there are a lot of work needs to be done to evaluate the exact therapeutic effects and mechanisms of those active ingredients, specifically, to establish a unified standard for diagnosis and evaluation of curative effect.”
“Depression is a widespread psychological disorder that affects up to 20% of the world’s population. Traditional Chinese medicine (TCM), with its unique curative effect in depression treatment, is gaining increasing attention as the discovery of novel antidepressant drug has become the pursuit of pharmaceutical. This article summarizes the work done on the natural products from TCM that have been reported to conceive antidepressant effects in the past two decades, which can be classified according to various mechanisms including increasing synaptic concentrations of monoamines, alleviating the hypothalamic-pituitary-adrenal (HPA) axis dysfunctions, lightening the impairment of neuroplasticity, fighting towards immune and inflammatory dysregulation. The antidepressant active ingredients identified can be generally divided into saponins, flavonoids, alkaloids, polysaccharides and others. Albiflorin, Baicalein, Berberine chloride, beta-Asarone, cannabidiol, Curcumin, Daidzein, Echinocystic acid (EA), Emodin, Ferulic acid, Gastrodin, Genistein, Ginsenoside Rb1, Ginsenoside Rg1, Ginsenoside Rg3, Hederagenin, Hesperidin, Honokiol, Hyperoside, Icariin, Isoliquiritin, Kaempferol, Liquiritin, L-theanine, Magnolol, Paeoniflorin, Piperine, Proanthocyanidin, Puerarin, Quercetin, Resveratrol (trans), Rosmarinic acid, Saikosaponin A, Senegenin, Tetrahydroxystilbene glucoside and Vanillic acid are Specified in this review. Simultaneously, chemical structures of the active ingredients with antidepressant activities are listed and their sources, models, efficacy and mechanisms are described. Chinese compound prescription and extracts that exert antidepressant effects are also introduced, which may serve as a source of inspiration for further development. In the view of present study, the antidepressant effect of certain TCMs are affirmative and encouraging. However, there are a lot of work needs to be done to evaluate the exact therapeutic effects and mechanisms of those active ingredients, specifically, to establish a unified standard for diagnosis and evaluation of curative effect.”


 “According to recent studies,
“According to recent studies, 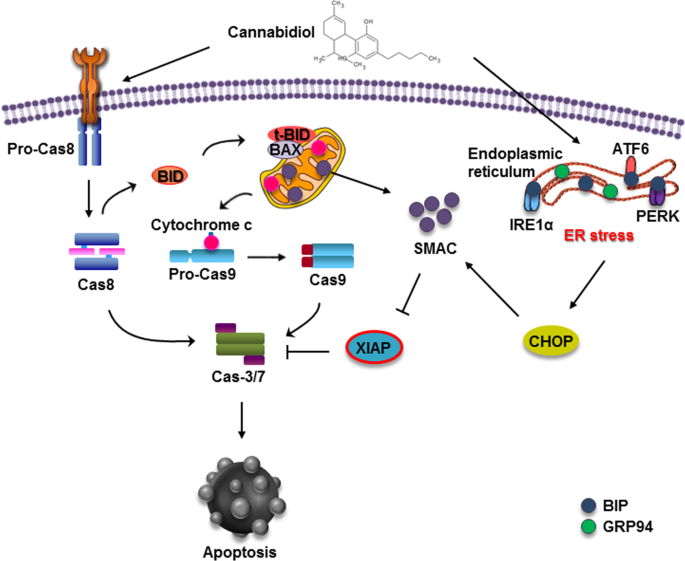
 “The healing properties of
“The healing properties of  “Cannabis use disorder (CUD) prevalence among people reporting past-year cannabis use declined from 2002–2016.
“Cannabis use disorder (CUD) prevalence among people reporting past-year cannabis use declined from 2002–2016. “Growing evidence suggests that medical marijuana laws have harm reduction effects across a variety of outcomes related to risky health behaviors. This study investigates the impact of medical marijuana laws on self-reported health using data from the Behavioral Risk Factor Surveillance System from 1993 to 2013. In our analyses we separately identify the effect of a medical marijuana law and the impact of subsequent active and legally protected dispensaries. Our main results show surprisingly limited improvements in self-reported health after the legalization of medical marijuana and legally protected dispensaries. Subsample analyses reveal strong improvements in health among non-white individuals, those reporting chronic pain, and those with a high school degree, driven predominately by whether or not the state had active and legally protected dispensaries. We also complement the analysis by evaluating the impact on risky health behaviors and find that the aforementioned demographic groups experience large reductions in alcohol consumption after the implementation of a medical marijuana law.”
“Growing evidence suggests that medical marijuana laws have harm reduction effects across a variety of outcomes related to risky health behaviors. This study investigates the impact of medical marijuana laws on self-reported health using data from the Behavioral Risk Factor Surveillance System from 1993 to 2013. In our analyses we separately identify the effect of a medical marijuana law and the impact of subsequent active and legally protected dispensaries. Our main results show surprisingly limited improvements in self-reported health after the legalization of medical marijuana and legally protected dispensaries. Subsample analyses reveal strong improvements in health among non-white individuals, those reporting chronic pain, and those with a high school degree, driven predominately by whether or not the state had active and legally protected dispensaries. We also complement the analysis by evaluating the impact on risky health behaviors and find that the aforementioned demographic groups experience large reductions in alcohol consumption after the implementation of a medical marijuana law.” “Memantine and marijuana smoking have been previously found to inhibit tremor in parkinsonian patients, however, the observed effects were relatively weak. The tremorolytic efficacy of memantine and
“Memantine and marijuana smoking have been previously found to inhibit tremor in parkinsonian patients, however, the observed effects were relatively weak. The tremorolytic efficacy of memantine and  “To evaluate interest in and patterns of use of non-prescription
“To evaluate interest in and patterns of use of non-prescription