 “Pancreatic cancer (PC) is related to lifestyle risks, chronic inflammation, and germline mutations in BRCA1/2, ATM, MLH1, TP53, or CDKN2A. Surgical resection and adjuvant chemotherapy are the main therapeutic strategies but are less effective in patients with high-grade tumors.
“Pancreatic cancer (PC) is related to lifestyle risks, chronic inflammation, and germline mutations in BRCA1/2, ATM, MLH1, TP53, or CDKN2A. Surgical resection and adjuvant chemotherapy are the main therapeutic strategies but are less effective in patients with high-grade tumors.
Oxygen-ozone (O2/O3) therapy is an emerging alternative tool for the treatment of several clinical disorders. O2/O3 therapy has been found to ameliorate mechanisms promoting chronic pain and inflammation, including hypoxia, inflammatory mediators, and infection.
The advantages of using cannabinoids have been evaluated in vitro and in vivo models of several human cancers. Regarding PDAC, activation of cannabinoid receptors was found to induce pancreatic cancer cell apoptosis without affecting the normal pancreas cells.
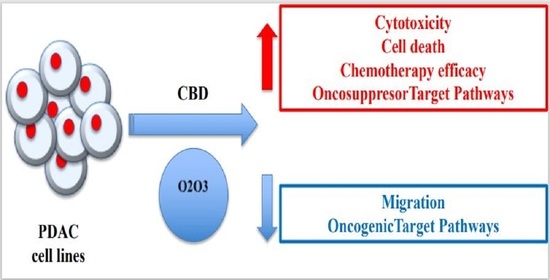
In a murine model of PDAC, a combination of cannabidiol (CBD) and gemcitabine increased survival length by nearly three times. Herein, we evaluate the anticancer effect of CBD and O2/O3, alone or in combination, on two human PDAC cell lines, PANC-1 and MiaPaCa-2, examining expression profiles of 92 pancreatic adenocarcinoma associated genes, cytotoxicity, migration properties, and cell death. Finally, we assess the combination effects with gemcitabine and paclitaxel.
Summarizing, for the first time the antitumoral effect of combined therapy with CBD and oxygen-ozone therapy in PDAC is evidenced.”
https://pubmed.ncbi.nlm.nih.gov/32992648/
https://www.mdpi.com/2072-6694/12/10/2774

 “Cannabinoids are increasingly-used substances in the treatment of chronic pain, some neuropsychiatric disorders and more recently, skin disorders with an inflammatory component.
“Cannabinoids are increasingly-used substances in the treatment of chronic pain, some neuropsychiatric disorders and more recently, skin disorders with an inflammatory component.





