“Introduction: Glioblastoma (GBM) is the most common invasive brain tumor composed of diverse cell types with poor prognosis. The highly complex tumor microenvironment (TME) and its interaction with tumor cells play important roles in the development, progression, and durability of GBM. Angiogenic and immune factors are two major components of TME of GBM; their interplay is a major determinant of tumor vascularization, immune profile, as well as immune unresponsiveness of GBM. Given the ineffectiveness of current standard therapies (surgery, radiotherapy, and concomitant chemotherapy) in managing patients with GBM, it is necessary to develop new ways of treating these lethal brain tumors. Targeting TME, altering tumor ecosystem may be a viable therapeutic strategy with beneficial effects for patients in their fight against GBM. Materials and Methods: Given the potential therapeutic effects of cannabidiol (CBD) in a wide spectrum of diseases, including malignancies, we tested, for the first time, whether inhalant CBD can inhibit GBM tumor growth using a well-established orthotopic murine model. Optical imaging, histology, immunohistochemistry, and flow cytometry were employed to describe the outcomes such as tumor progression, cancer cell signaling pathways, and the TME. Results: Our findings showed that inhalation of CBD was able to not only limit the tumor growth but also to alter the dynamics of TME by repressing P-selectin, apelin, and interleukin (IL)-8, as well as blocking a key immune checkpoint-indoleamine 2,3-dioxygenase (IDO). In addition, CBD enhanced the cluster of differentiation (CD) 103 expression, indicating improved antigen presentation, promoted CD8 immune responses, and reduced innate Lymphoid Cells within the tumor. Conclusion: Overall, our novel findings support the possible therapeutic role of inhaled CBD as an effective, relatively safe, and easy to administer treatment adjunct for GBM with significant impacts on the cellular and molecular signaling of TME, warranting further research.”
Category Archives: Brain Cancer
The Endocannabinoid System: A Potential Target for the Treatment of Various Diseases
 “The Endocannabinoid System (ECS) is primarily responsible for maintaining homeostasis, a balance in internal environment (temperature, mood, and immune system) and energy input and output in living, biological systems.
“The Endocannabinoid System (ECS) is primarily responsible for maintaining homeostasis, a balance in internal environment (temperature, mood, and immune system) and energy input and output in living, biological systems.
In addition to regulating physiological processes, the ECS directly influences anxiety, feeding behaviour/appetite, emotional behaviour, depression, nervous functions, neurogenesis, neuroprotection, reward, cognition, learning, memory, pain sensation, fertility, pregnancy, and pre-and post-natal development.
The ECS is also involved in several pathophysiological diseases such as cancer, cardiovascular diseases, and neurodegenerative diseases. In recent years, genetic and pharmacological manipulation of the ECS has gained significant interest in medicine, research, and drug discovery and development.
The distribution of the components of the ECS system throughout the body, and the physiological/pathophysiological role of the ECS-signalling pathways in many diseases, all offer promising opportunities for the development of novel cannabinergic, cannabimimetic, and cannabinoid-based therapeutic drugs that genetically or pharmacologically modulate the ECS via inhibition of metabolic pathways and/or agonism or antagonism of the receptors of the ECS. This modulation results in the differential expression/activity of the components of the ECS that may be beneficial in the treatment of a number of diseases.
This manuscript in-depth review will investigate the potential of the ECS in the treatment of various diseases, and to put forth the suggestion that many of these secondary metabolites of Cannabis sativa L. (hereafter referred to as “C. sativa L.” or “medical cannabis”), may also have potential as lead compounds in the development of cannabinoid-based pharmaceuticals for a variety of diseases.”
https://www.mdpi.com/1422-0067/22/17/9472
“Cannabis sativa L. as a Natural Drug Meeting the Criteria of a Multitarget Approach to Treatment”
Cannabidiol Induces Apoptosis and Perturbs Mitochondrial Function in Human and Canine Glioma Cells
 “Cannabidiol (CBD), the major non-psychoactive compound found in cannabis, is frequently used both as a nutraceutical and therapeutic.
“Cannabidiol (CBD), the major non-psychoactive compound found in cannabis, is frequently used both as a nutraceutical and therapeutic.
Despite anecdotal evidence as an anticancer agent, little is known about the effect CBD has on cancer cells. Given the intractability and poor prognoses of brain cancers in human and veterinary medicine, we sought to characterize the in vitro cytotoxicity of CBD on human and canine gliomas.
Glioma cells treated with CBD showed a range of cytotoxicity from 4.9 to 8.2 μg/ml; canine cells appeared to be more sensitive than human.
These results demonstrate the cytotoxic nature of CBD in human and canine glioma cells and suggest a mechanism of action involving dysregulation of calcium homeostasis and mitochondrial activity.”
“In this present study, we demonstrate that highly purified CBD isolate reduced proliferation and induced caspase-mediated cell death, suggestive of apoptosis, in both canine glioma cell lines SDT3G and J3TBG as well as the human glioma cell lines U87MG and U373MG Uppsala. The growing body of knowledge of the pharmacology, anticancer effects, and other therapeutically relevant properties of cannabidiol reveal the exciting potential of CBD as a potential clinical therapeutic.”
https://www.frontiersin.org/articles/10.3389/fphar.2021.725136/full
Cancer Initiation, Progression and Resistance: Are Phytocannabinoids from Cannabis sativa L. Promising Compounds?
 “Cannabis sativa L. is a source of over 150 active compounds known as phytocannabinoids that are receiving renewed interest due to their diverse pharmacologic activities. Indeed, phytocannabinoids mimic the endogenous bioactive endocannabinoids effects through activation of CB1 and CB2 receptors widely described in the central nervous system and peripheral tissues.
“Cannabis sativa L. is a source of over 150 active compounds known as phytocannabinoids that are receiving renewed interest due to their diverse pharmacologic activities. Indeed, phytocannabinoids mimic the endogenous bioactive endocannabinoids effects through activation of CB1 and CB2 receptors widely described in the central nervous system and peripheral tissues.
All phytocannabinoids have been studied for their protective actions towards different biological mechanisms, including inflammation, immune response, oxidative stress that, altogether, result in an inhibitory activity against the carcinogenesis.
The role of the endocannabinoid system is not yet completely clear in cancer, but several studies indicate that cannabinoid receptors and endogenous ligands are overexpressed in different tumor tissues.
Recently, in vitro and in vivo evidence support the effectiveness of phytocannabinoids against various cancer types, in terms of proliferation, metastasis, and angiogenesis, actions partially due to their ability to regulate signaling pathways critical for cell growth and survival.
The aim of this review was to report the current knowledge about the action of phytocannabinoids from Cannabis sativa L. against cancer initiation and progression with a specific regard to brain, breast, colorectal, and lung cancer as well as their possible use in the therapies. We will also report the known molecular mechanisms responsible for such positive effects.
Finally, we will describe the actual therapeutic options for Cannabis sativa L. and the ongoing clinical trials.”
https://www.mdpi.com/1420-3049/26/9/2668
A Phase 2 Randomised Clinical Trial Assessing the Tolerability of Two Different Ratios of Medicinal Cannabis in Patients With High Grade Gliomas
 “Background: Cannabis for cancer is very topical and, given the use of illicit cannabis preparations used in this vulnerable population, research investigating standardised, quality-assured medicinal cannabis is critical to inform clinicians and assist patient safety.
“Background: Cannabis for cancer is very topical and, given the use of illicit cannabis preparations used in this vulnerable population, research investigating standardised, quality-assured medicinal cannabis is critical to inform clinicians and assist patient safety.
Methods: A randomized trial involving adult patients diagnosed with a high-grade glioma, no history of substance abuse, liver or kidney damage or myocardial infarction were eligible for inclusion in a tolerability study on two different ratios of medicinal cannabis. Baseline screening of brain morphology, blood pathology, functional status, and cognition was conducted. A retrospective control group was used for comparison for secondary outcomes.
Results: Participants (n=88) were on average 53.3 years old. A paired t-test assessed the Functional Assessment of Cancer Therapy for Brain Cancer (FACT-Br) between groups from baseline to week 12 found that the 1:1 ratio favoured both physical (p=0.025) and functional (p=0.014) capacity and improved sleep (p=0.009). Analysis of changes from baseline to week 12 also found 11% of 61 participants had a reduction in disease, 34% were stable, 16% had slight enhancement, and 10% had progressive disease. No serious adverse events occurred. Side effects included dry mouth, tiredness at night, dizziness, drowsiness.
Conclusion: This study demonstrated that a single nightly dose of THC-containing medicinal cannabis was safe, had no serious adverse effects and was well tolerated in patients. Medicinal cannabis significantly improved sleep, functional wellbeing, and quality of life.”
https://pubmed.ncbi.nlm.nih.gov/34094937/
“From this study we have shown that a single nightly dose of THC-containing cannabis was well tolerated in patients in both groups with high-grade gliomas and significantly improved sleep, functional wellbeing and contentment with QoL in a sample of patients compared to baseline. From this trial, the 1:1 ratio has been identified as the preferred combination the moving forward to further trials. This study significantly informs MC product choice for ongoing studies into cannabis being a potential adjunct treatment option for this patient population.”
https://www.frontiersin.org/articles/10.3389/fonc.2021.649555/full
Specific Compositions of Cannabis sativa Compounds Have Cytotoxic Activity and Inhibit Motility and Colony Formation of Human Glioblastoma Cells In Vitro
 “Glioblastoma multiforme (GBM) is the most lethal subtype of glioma. Cannabis sativa is used for the treatment of various medical conditions. Around 150 phytocannabinoids have been identified in C. sativa, among them Δ-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) that trigger GBM cell death. However, the optimal combinations of cannabis molecules for anti-GBM activity are unknown. Chemical composition was determined using high-performance liquid chromatography (HPLC) and gas chromatography mass spectrometry (GC/MS). Cytotoxic activity was determined by XTT and lactate dehydrogenase (LDH) assays and apoptosis and cell cycle by fluorescence-activated cell sorting (FACS). F-actin structures were observed by confocal microscopy, gene expression by quantitative PCR, and cell migration and invasion by scratch and transwell assays, respectively. Fractions of a high-THC cannabis strain extract had significant cytotoxic activity against GBM cell lines and glioma stem cells derived from tumor specimens. A standard mix (SM) of the active fractions F4 and F5 induced apoptosis and expression of endoplasmic reticulum (ER)-stress associated-genes. F4 and F5 inhibited cell migration and invasion, altered cell cytoskeletons, and inhibited colony formation in 2 and 3-dimensional models. Combinations of cannabis compounds exert cytotoxic, anti-proliferative, and anti-migratory effects and should be examined for efficacy on GBM in pre-clinical studies and clinical trials.”
“Glioblastoma multiforme (GBM) is the most lethal subtype of glioma. Cannabis sativa is used for the treatment of various medical conditions. Around 150 phytocannabinoids have been identified in C. sativa, among them Δ-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) that trigger GBM cell death. However, the optimal combinations of cannabis molecules for anti-GBM activity are unknown. Chemical composition was determined using high-performance liquid chromatography (HPLC) and gas chromatography mass spectrometry (GC/MS). Cytotoxic activity was determined by XTT and lactate dehydrogenase (LDH) assays and apoptosis and cell cycle by fluorescence-activated cell sorting (FACS). F-actin structures were observed by confocal microscopy, gene expression by quantitative PCR, and cell migration and invasion by scratch and transwell assays, respectively. Fractions of a high-THC cannabis strain extract had significant cytotoxic activity against GBM cell lines and glioma stem cells derived from tumor specimens. A standard mix (SM) of the active fractions F4 and F5 induced apoptosis and expression of endoplasmic reticulum (ER)-stress associated-genes. F4 and F5 inhibited cell migration and invasion, altered cell cytoskeletons, and inhibited colony formation in 2 and 3-dimensional models. Combinations of cannabis compounds exert cytotoxic, anti-proliferative, and anti-migratory effects and should be examined for efficacy on GBM in pre-clinical studies and clinical trials.”
“Glioblastoma multiforme (GBM) is the most frequent, invasive, and lethal subtype of glioma brain tumors. Cannabis is commonly used for medical treatment, and individual phytocannabinoids have been shown to trigger GBM cell death. However, cannabis contains hundreds of different compounds, and the optimal combinations of molecules with anti-GBM activity are unknown. Here, we identified fractions from a cannabis strain that substantially reduced human GBM cell viability and motility. The fractions also reduced the ability of GBM cells to form colonies in 2 and 3-dimensional models, suggesting that the cannabis treatments may have the potential for preventing the formation of GBM neurospheres associated with the high resistance to current therapies. Importantly, these compounds also induced cell death in glioma stem cells derived from tumor specimens. The effectiveness of the fractions and combinations of cannabis compounds should be examined in GBM pre-clinical studies and clinical trials.”
Cannabidiol converts NFκB into a tumor suppressor in glioblastoma with defined antioxidative properties
“The transcription factor NFκB drives neoplastic progression of many cancers including primary brain tumors (glioblastoma; GBM). Precise therapeutic modulation of NFκB activity can suppress central oncogenic signalling pathways in GBM, but clinically applicable compounds to achieve this goal have remained elusive.
Methods: In a pharmacogenomics study with a panel of transgenic glioma cells we observed that NFκB can be converted into a tumor suppressor by the non-psychotropic cannabinoid Cannabidiol (CBD). Subsequently, we investigated the anti-tumor effects of CBD, which is used as an anticonvulsive drug (Epidiolex) in pediatric neurology, in a larger set of human primary GBM stem-like cells (hGSC). For this study we performed pharmacological assays, gene expression profiling, biochemical and cell-biological experiments. We validated our findings using orthotopic in vivo models and bioinformatics analysis of human GBM-datasets.
Results: We found that CBD promotes DNA binding of the NFκB subunit RELA and simultaneously prevents RELA-phosphorylation on serine-311, a key residue which permits genetic transactivation. Strikingly, sustained DNA binding by RELA lacking phospho-serine 311 was found to mediate hGSC cytotoxicity. Widespread sensitivity to CBD was observed in a cohort of hGSC defined by low levels of reactive oxygen-species (ROS), while high ROS-content in other tumors blocked CBD induced hGSC death. Consequently, ROS levels served as predictive biomarker for CBD-sensitive tumors.
Conclusions: This evidence demonstrates how a clinically approved drug can convert NFκB into a tumor suppressor and suggests a promising repurposing option for GBM-therapy.”
https://pubmed.ncbi.nlm.nih.gov/33864076/
https://academic.oup.com/neuro-oncology/advance-article/doi/10.1093/neuonc/noab095/6231710
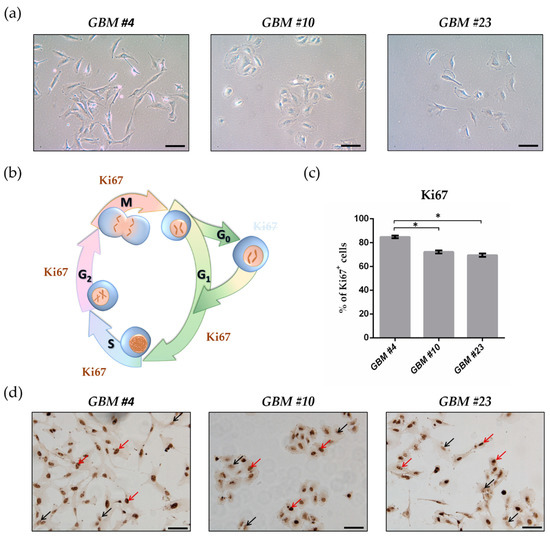
THC Reduces Ki67-Immunoreactive Cells Derived from Human Primary Glioblastoma in a GPR55-Dependent Manner

“Glioblastoma (GBM) is the most frequent malignant tumor of the central nervous system in humans with a median survival time of less than 15 months.
∆9-Tetrahydrocannabinol (THC) and cannabidiol (CBD) are the best-characterized components of Cannabis sativa plants with modulating effects on cannabinoid receptors 1 and 2 (CB1 and CB2) and on orphan receptors such as GPR18 or GPR55. Previous studies have demonstrated anti-tumorigenic effects of THC and CBD in several tumor entities including GBM, mostly mediated via CB1 or CB2.
In this study, we investigated the non-CB1/CB2 effects of THC on the cell cycle of GBM cells isolated from human tumor samples.
Cell cycle entry was measured after 24 h upon exposure by immunocytochemical analysis of Ki67 as proliferation marker. The Ki67-reducing effect of THC was abolished in the presence of CBD, whereas CBD alone did not cause any changes. To identify the responsible receptor for THC effects, we first characterized the cells regarding their expression of different cannabinoid receptors: CB1, CB2, GPR18, and GPR55. Secondly, the receptors were pharmacologically blocked by application of their selective antagonists AM281, AM630, O-1918, and CID16020046 (CID), respectively. All examined cells expressed the receptors, but only in presence of the GPR55 antagonist CID was the THC effect diminished. Stimulation with the GPR55 agonist lysophosphatidylinositol (LPI) revealed similar effects as obtained for THC. The LPI effects were also inhibited by CBD and CID, confirming a participation of GPR55 and suggesting its involvement in modifying the cell cycle of patient-derived GBM cells.”
https://pubmed.ncbi.nlm.nih.gov/33802282/
https://www.mdpi.com/2072-6694/13/5/1064
Efficacy of cannabinoids against glioblastoma multiforme: A systematic review

“INTRODUCTION
: The increased incidence of Glioblastoma Multiforme, the most aggressive and most common primary brain tumour, is evident worldwide. Survival rates are reaching only 15 months due to its high recurrence and resistance to current combination therapies including oncotomy, radiotherapy and chemotherapy. Light has been shed in the recent years on the anticancer properties of cannabinoids from Cannabis sativa.
OBJECTIVE
: To determine whether cannabinoids alone or in combination with radiotherapy and/or chemotherapy inhibit tumour progression, induce cancer cell death, inhibit metastasis and invasiveness and the mechanisms that underlie these actions.
METHOD
: PubMed and Web of Science were used for a systemic search to find studies on the anticancer effects of natural cannabinoids on glioma cancer cells in vitro and/or in vivo.
RESULTS
: A total of 302 papers were identified, of which 14 studies were found to fit the inclusion criteria. 5 studies were conducted in vitro, 2 in vivo and 7 were both in vivo and in vitro. 3 studies examined the efficacy of CBD, THC and TMZ, 1 study examined CBD and radiation, 2 studies examined efficacy of THC only and 3 studies examined the efficacy of CBD only. 1 study examined the efficacy of CBD, THC and radiotherapy, 2 studies examined the combination of CBD and THC and 2 more studies examined the efficacy of CBD and TMZ.
CONCLUSION
: The evidence in this systematic review leads to the conclusion that cannabinoids possess anticancer potencies against glioma cells, however this effect varies with the combinations and dosages used. Studies so far were conducted on cells in culture and on mice as well as a small number of studies that were conducted on humans. Hence in order to have more accurate results, higher quality studies mainly including human clinical trials with larger sample sizes are necessitated urgently for GBM treatment.”
HTTPS://WWW.SCIENCEDIRECT.COM/SCIENCE/ARTICLE/ABS/PII/S0944711321000751
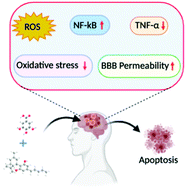
A cannabidiol-loaded Mg-gallate metal-organic framework-based potential therapeutic for glioblastomas

In the present study, we explored the anticancer effect of cannabidiol loaded magnesium-gallate (CBD/Mg-GA) metal-organic framework (MOF) using the rat glioma brain cancer (C6) cell line.
Bioactive and microporous magnesium gallate MOF was employed for simultaneous delivery of two potential anticancer agents (gallic acid and CBD) to the cancer cells. Gallic acid (GA), a polyphenolic compound, is part of the MOF framework, while CBD is loaded within the framework. Slow degradation of CBD/Mg-GA MOF in physiological fluids leads to sustained release of GA and CBD.
CBD’s anti-cancer actions target mitochondria, inducing their dysfunction and generation of harmful reactive oxygen species (ROS). Anticancer effects of CBD/Mg-GA include a significant increase in ROS production and a reduction in anti-inflammatory responses as reflected by a significant decrease in TNF-α expression levels. Molecular mechanisms that underlie these effects include the modulation of NF-κB expression, triggering the apoptotic cascades of glioma cells. CBD/Mg-GA MOF has potential anti-cancer, anti-inflammatory and anti-oxidant properties.
Thus, the present study demonstrates that CBD/Mg-GA MOF may be a promising therapeutic for glioblastoma.”
https://pubmed.ncbi.nlm.nih.gov/33657198/
https://pubs.rsc.org/en/content/articlelanding/2021/TB/D0TB02780D#!divAbstract