Category Archives: Uncategorized
Cannabinoids for skin diseases and hair regrowth
 “The use of cannabis for skin diseases and hair regrowth is at the preliminary stage.
“The use of cannabis for skin diseases and hair regrowth is at the preliminary stage.
Legalization: Many countries have approved cannabis for medical use; however, four countries Canada, Uruguay, South Africa, and Georgia have legalized it for both medical and recreational purposes.
The endocannabinoid system: The endocannabinoid system may maintain skin homeostasis; two notable endocannabinoids include 2-Arachidonoylglycerol (2-AG) and N-arachidonoylethanolamine (AEA).
Routes of administration and pharmacokinetics: Topical cannabinoids can avoid the first-pass metabolism and reduce respiratory side effects; however, the high hydrophobicity of cannabinoids may hinder percutaneous absorption.
Skin disorders and hair growth: Human clinical studies suggest that cannabinoids may be used in eczema, acne, pruritus, and systemic sclerosis treatment. Cannabidiol (CBD) may enhance hair growth via multiple mechanisms.
Safety: Topical cannabis may cause mild side effects such as pruritus, burning, erythema, and stinging; they are relatively safer than inhalation and oral cannabis. Cannabis use may be associated with allergic symptoms and reduced immune response to live vaccination.
Cannabinoids in practice: Despite growing interest, dermatologists should be cautious prescribing cannabinoids due to insufficient clinical data on both efficacy and safety.”
https://pubmed.ncbi.nlm.nih.gov/34363728/
https://onlinelibrary.wiley.com/doi/10.1111/jocd.14352
The Therapeutic Potential of Cannabis in Counteracting Oxidative Stress and Inflammation
 “Significant growth of interest in cannabis (Cannabis sativa L.), especially its natural anti-inflammatory and antioxidative properties, has been observed recently. This narrative review aimed to present the state of the art of research concerning the anti-inflammatory activity of all classes of cannabinoids published in the last five years. Multimodal properties of cannabinoids include their involvement in immunological processes, anti-inflammatory, and antioxidative effects. Cannabinoids and non-cannabinoid compounds of cannabis proved their anti-inflammatory effects in numerous animal models. The research in humans is missing, and the results are unconvincing. Although preclinical evidence suggests cannabinoids are of value in treating chronic inflammatory diseases, the clinical evidence is scarce, and further well-designed clinical trials are essential to determine the prospects for using cannabinoids in inflammatory conditions.”
“Significant growth of interest in cannabis (Cannabis sativa L.), especially its natural anti-inflammatory and antioxidative properties, has been observed recently. This narrative review aimed to present the state of the art of research concerning the anti-inflammatory activity of all classes of cannabinoids published in the last five years. Multimodal properties of cannabinoids include their involvement in immunological processes, anti-inflammatory, and antioxidative effects. Cannabinoids and non-cannabinoid compounds of cannabis proved their anti-inflammatory effects in numerous animal models. The research in humans is missing, and the results are unconvincing. Although preclinical evidence suggests cannabinoids are of value in treating chronic inflammatory diseases, the clinical evidence is scarce, and further well-designed clinical trials are essential to determine the prospects for using cannabinoids in inflammatory conditions.”
https://www.mdpi.com/1420-3049/26/15/4551
Myrcene-What Are the Potential Health Benefits of This Flavouring and Aroma Agent?
“Myrcene (β-myrcene) is an abundant monoterpene which occurs as a major constituent in many plant species, including hops and cannabis. It is a popular flavouring and aroma agent (food additive) used in the manufacture of food and beverages. This review aims to report on the occurrence, biological and toxicological profile of β-myrcene. The main reported biological properties of β-myrcene-anxiolytic, antioxidant, anti-ageing, anti-inflammatory, analgesic properties-are discussed, with the mechanisms of activity. Here we also discuss recent data regarding the safety of β-myrcene. Overall, β-myrcene has shown promising health benefits in many animal studies. However, studies conducted in humans is lacking. In the future, there is potential for the formulation and production of non-alcoholic beers, functional foods and drinks, and cannabis extracts (low in THC) rich in β-myrcene.”
“β-Myrcene characteristically gives cannabis strains a mildly sweet flavour profile and provides scent notes that are spicy, earthy and musky. Cannabis strains which contain high concentrations of myrcene (>0.5% myrcene), are likely to induce sedative qualities (“couch-lock effect”), which are classically attributed to Cannabis indica Lam (a synonym of C. sativa L.) strains. On the other hand, strains low in β-myrcene (<0.5%) are likely to induce a more energic “high”.β-Myrcene reported biological activities include analgesic, sedative, antidiabetic, antioxidant, anti-inflammatory, antibacterial, and anticancer effects.”
https://www.frontiersin.org/articles/10.3389/fnut.2021.699666/full
A pilot safety, tolerability and pharmacokinetic study of an oro-buccal administered cannabidiol-dominant anti-inflammatory formulation in healthy individuals: a randomized placebo-controlled single-blinded study
 “Background: The cannabis plant presents a complex biochemical unit of over 500 constituents of which 70 or more molecules have been classified as cannabinoids binding to cannabinoid receptors. The study aimed to investigate the safety, tolerability, and preliminary pharmacokinetics of a nanoparticle CBD formulation.
“Background: The cannabis plant presents a complex biochemical unit of over 500 constituents of which 70 or more molecules have been classified as cannabinoids binding to cannabinoid receptors. The study aimed to investigate the safety, tolerability, and preliminary pharmacokinetics of a nanoparticle CBD formulation.
Results: The study met the primary outcomes of safety, tolerability, and preliminary pharmacokinetics of a standardized CBD-dominant anti-inflammatory extract for oro-buccal administration. Bioavailability of a 6 mg and 18 mg dose of CBD (median IQR) was 0.87 and 8.9 ng h mL-1, respectively. The maximum concentration of CBD for the low and high doses administered once per day occurred at 60 min for both concentrations. The median half-life of the 6 mg and 18 mg CBD dose was 1.23 and 5.45 h, respectively. The apparent clearance of CBD was 115 and 34 L min-1 for a 6 mg and 18 mg dose, respectively.
Conclusion: The oro-buccal nanoparticle formulation achieved plasma concentrations that were largely comparable to other commercial and investigated formulations relative to the concentrations administered. Moreover, there were no reports of adverse effects associated with unfavorable inflammatory sequalae.”
https://link.springer.com/article/10.1007%2Fs10787-021-00859-y
The Effects of Cannabis sativa L. Extract on Oxidative Stress Markers In Vivo
 “In recent decades, a lot of attention has been paid to Cannabis sativa L. due to its useful applications, including in fibers, oil, food for humans and animals, and therapeutics.
“In recent decades, a lot of attention has been paid to Cannabis sativa L. due to its useful applications, including in fibers, oil, food for humans and animals, and therapeutics.
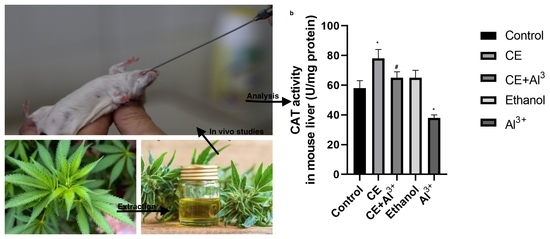
The present study aimed to determine antioxidant activity of cannabinoids in Cannabis sativa L. in vivo, evaluating the possible antioxidative effect of Cannabis sativa L. extract (CE) on malondialdehyde (MDA) and glutathione (GSH) concentrations as well as on catalase (CAT) activity in BALB/c mice.
The findings in vivo indicate that Cannabis sativa L. is a good source of natural antioxidants and can be used in the management of oxidative stress.”
https://pubmed.ncbi.nlm.nih.gov/34357019/
https://www.mdpi.com/2075-1729/11/7/647
Association Between Smoking Cannabis and Quitting Cigarettes in a Large American Cancer Society Cohort
 “Background: Cannabis use is increasing, including among smokers, an at-risk population for cancer. Research is equivocal on whether using cannabis inhibits quitting cigarettes. The current longitudinal study investigated associations between smoking cannabis and subsequently quitting cigarettes.
“Background: Cannabis use is increasing, including among smokers, an at-risk population for cancer. Research is equivocal on whether using cannabis inhibits quitting cigarettes. The current longitudinal study investigated associations between smoking cannabis and subsequently quitting cigarettes.
Results: Adjusted cigarette quitting rates at follow-up did not differ significantly by baseline cannabis smoking status [never 36.2%, 95% confidence interval (CI), 34.5%-37.8%; former 34.1%, CI, 31.4%-37.0%; recent 33.6%, CI, 30.1%-37.3%], nor by frequency of cannabis smoking (low 31.4%, CI, 25.6%-37.3%; moderate 36.7%, CI, 30.7%-42.3%; high 34.4%, CI, 28.3%-40.2%) among recent baseline cannabis smokers. In cross-sectional analyses conducted at follow-up the proportion of cigarette smokers intending to quit smoking cigarettes in the next 30 days did not differ by cannabis smoking status (p=0.83).
Conclusions: Results do not support the hypothesis that cannabis smoking inhibits quitting cigarette smoking among adults.”
https://pubmed.ncbi.nlm.nih.gov/34348959/
“Results do not support the hypothesis that cannabis smoking inhibits quitting cigarette smoking among adults.” https://cebp.aacrjournals.org/content/early/2021/08/04/1055-9965.EPI-20-1810
Cannabis Improves Stuttering: Case Report and Interview with the Patient
 “Introduction: Speech dysfluency, often referred to as stuttering, is a frequent speech disorder encountered in about 5% of children. Although in the majority of people affected, symptoms improve in adulthood, in some patients, stuttering persists and significantly impairs everyday functioning and quality of life. Treatment for stuttering includes speech therapy, cognitive behavioral therapy, and relaxation techniques. However, a substantial number of patients do not benefit sufficiently from these treatment strategies or are even treatment resistant.
“Introduction: Speech dysfluency, often referred to as stuttering, is a frequent speech disorder encountered in about 5% of children. Although in the majority of people affected, symptoms improve in adulthood, in some patients, stuttering persists and significantly impairs everyday functioning and quality of life. Treatment for stuttering includes speech therapy, cognitive behavioral therapy, and relaxation techniques. However, a substantial number of patients do not benefit sufficiently from these treatment strategies or are even treatment resistant.
Methods: We present the case of a 20-year-old male with treatment-resistant stuttering, who markedly improved after treatment with medicinal cannabis.
Results: Besides improved speech fluency as assessed by several phoniatric tests, we observed remission of (social) anxiety, improved mood, and reduced stress, resulting in an overall improvement of quality of life after cannabis therapy. The patient, in addition, reported improved attention, concentration, and sleep, increased self-confidence, and better social life. No side effects occurred. Over a time period of more than a year, treatment was equally effective. In an interview, the patient describes his personal view and the influence of cannabis-based treatment on his life.
Conclusions: Medicinal cannabis could be effective in treatment of refractory stuttering, but these preliminary data have to be confirmed in controlled studies.”
Use and caregiver-reported efficacy of medical cannabis in children and adolescents in Switzerland
 “Evidence on the use and efficacy of medical cannabis for children is limited. We examined clinical and epidemiological characteristics of medical cannabis treatment and caregiver-reported effects in children and adolescents in Switzerland.
“Evidence on the use and efficacy of medical cannabis for children is limited. We examined clinical and epidemiological characteristics of medical cannabis treatment and caregiver-reported effects in children and adolescents in Switzerland.
We collected clinical data from children and adolescents (< 18 years) who received Δ9-tetrahydrocannabinol (THC), cannabidiol (CBD), or a combination of the two between 2008 and 2019 in Switzerland. Out of 205 contacted families, 90 agreed to participate. The median age at the first prescription was 11.5 years (interquartile range (IQR) 6-16), and 32 patients were female (36%). Fifty-one (57%) patients received CBD only and 39 (43%) THC. Patients were more likely to receive THC therapy if one of the following symptoms or signs were present: spasticity, pain, lack of weight gain, vomiting, or nausea, whereas seizures were the dominant indication for CBD therapy.
Improvements were reported in 59 (66%) study participants.
The largest treatment effects were reported for pain, spasticity, and frequency of seizures in participants treated with THC, and for those treated with pure CBD, the frequency of seizures. However, 43% of caregivers reported treatment interruptions, mainly because of lack of improvement (56%), side effects (46%), the need for a gastric tube (44%), and cost considerations (23%).
Conclusions: The effects of medical cannabis in children and adolescents with chronic conditions are unknown except for rare seizure disorders, but the caregiver-reported data analysed here may justify trials of medical cannabis with standardized concentrations of THC or CBD to assess its efficacy in the young.
What is Known: • The use of medical cannabis (THC and CBD) to treat a variety of diseases among children and adolescents is increasing. • In contrast to adults, there is no evidence to support the use of medical cannabis to treat chronic pain and spasticity in children, but substantial evidence to support the use of CBD in children with rare seizure disorders.
What is New: • This study provides important insights into prescription practices, dosages, and treatment outcomes in children and adolescents using medical cannabis data from a real-life setting.
• The effects of medical cannabis in children and adolescents with chronic conditions shown in our study support trials of medical cannabis for chronic conditions.”
“For two thirds of participants treated with standardized THC or CBD preparations, the caregiver reported an improvement in their condition and well-being. Medical cannabis could be a promising and useful therapy for children and adolescents with neurological conditions.”
https://link.springer.com/article/10.1007%2Fs00431-021-04202-z
Use of complementary therapies for chronic pain management in patients with reported Ehlers-Danlos syndrome or hypermobility spectrum disorders
 “Ehlers-Danlos Syndromes (EDS) and related Hypermobility Spectrum Disorders (HSD) are debilitating connective tissue disorders that feature a prominent pain component for which there are limited therapeutic options for pain management.
“Ehlers-Danlos Syndromes (EDS) and related Hypermobility Spectrum Disorders (HSD) are debilitating connective tissue disorders that feature a prominent pain component for which there are limited therapeutic options for pain management.
Consequently, many patients try various non-prescribed treatments, including complementary and alternative therapies that have not been well studied in the EDS/HSD patient population. We surveyed over 500 individuals through the EDS Society who reported having been diagnosed with EDS or HSD to ascertain what complementary and alternative therapies were used and their reported effectiveness in alleviating pain and improving quality of life.
Specifically, we focused on the use of traditional Chinese therapies, herbal medications, and marijuana.
The most commonly reported therapies, used by 70-92% of participants, were non-steroidal anti-inflammatory drugs, acetaminophen, opioids, and physical therapy.
Therapies rated by participants as most efficacious were opioids, physical therapy, and marijuana with 10-24% of those using these therapies rating them as extremely helpful.
Patient-initiated complementary therapy use in EDS/HSD patients is widespread at 56%. Complementary therapies were largely utilized by EDS/HSD patients with higher reported pain levels. Providers caring for EDS/HSD patients should be aware of these data showing broad usage of predominantly non-prescribed therapies and be prepared to consider such usage in working collaboratively with these patients to develop comprehensive treatment plans to manage their chronic pain complications.”

 “Oral ulcer is a common oral inflammatory lesion accompanied by severe pain but with few effective treatments. Cannabidiol (CBD) is recently emerging for its therapeutic potential in a range of diseases, including inflammatory conditions and cancers.
“Oral ulcer is a common oral inflammatory lesion accompanied by severe pain but with few effective treatments. Cannabidiol (CBD) is recently emerging for its therapeutic potential in a range of diseases, including inflammatory conditions and cancers.