 “Exemestane is one of the aromatase inhibitors (AI) used as first line treatment for estrogen-receptor positive breast cancer in post-menopausal women. Exemestane acts by inhibiting aromatase, the enzyme responsible for the conversion of androgens to estrogens and also by promoting apoptosis of breast cancer cells. Nevertheless, despite its therapeutic success, this AI, after prolonged treatment, can induce acquired resistance, which causes tumor relapse. Therefore, it is important to find new strategies to overcome resistance in order to improve breast cancer treatment.
“Exemestane is one of the aromatase inhibitors (AI) used as first line treatment for estrogen-receptor positive breast cancer in post-menopausal women. Exemestane acts by inhibiting aromatase, the enzyme responsible for the conversion of androgens to estrogens and also by promoting apoptosis of breast cancer cells. Nevertheless, despite its therapeutic success, this AI, after prolonged treatment, can induce acquired resistance, which causes tumor relapse. Therefore, it is important to find new strategies to overcome resistance in order to improve breast cancer treatment.
Considering that the development of resistance is the main reason for endocrine treatment failure, our group decided to explore the ability of three cannabinoids, Δ9-tetrahydrocannabinol (THC), cannabidiol (CBD) and anandamide (AEA), to reverse resistance to exemestane. The THC and CBD are phytocannabinoids derived from the plant Cannabis sativa (marijuana) whereas AEA is an endocannabinoid. For that, it was used LTEDaro cells, a long-term estrogen deprived ER+ breast cancer cell line that mimics resistance to exemestane. These cells were treated with exemestane in combination with two phytocannabinoids, CBD and THC, and the endocannabinoid AEA.
The presence of CB1 and CB2 in LTEDaro cells was confirmed by Western blot analysis and the effects of the combination of cannabinoids with exemestane were evaluated by MTT and LDH assays. Cell morphology was analyzed by Giemsa and Hoechst staining.
Results: Our results demonstrate that all the cannabinoids induce a decrease in viability of exemestane-resistant cells, in a dose- and time-dependent manner, without LDH release. These results indicate that the studied cannabinoids, mainly THC and AEA, revert the resistance to exemestane, probably by inducing apoptosis, as observed in Giemsa/Hoechst stain by the presence of typical morphological features of apoptosis.
Conclusion: This study highlights the efficacy of using cannabinoids as a potential adjuvant treatment to revert resistance to AIs.”
https://www.ncbi.nlm.nih.gov/pubmed/32258721
https://journals.lww.com/pbj/fulltext/2017/09000/The_effects_of_cannabinoids_in.118.aspx

 “Cannabis has been used for thousands of years in many cultures for the treatment of several ailments including pain.
“Cannabis has been used for thousands of years in many cultures for the treatment of several ailments including pain. “Medicinal use of
“Medicinal use of  “Since antiquity, Cannabis has provoked enormous intrigue for its potential medicinal properties as well as for its unique pharmacological effects.
“Since antiquity, Cannabis has provoked enormous intrigue for its potential medicinal properties as well as for its unique pharmacological effects.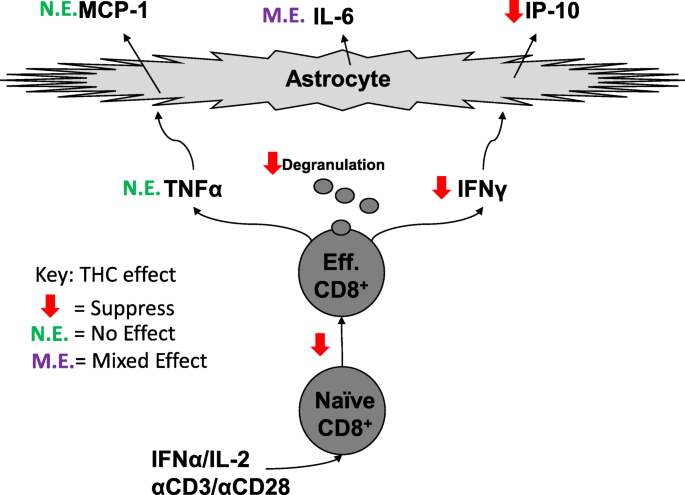 “CD8+ T cells can contribute to neuroinflammation by secretion of inflammatory cytokines like interferon γ (IFNγ) and tumor necrosis factor α (TNFα). Astrocytes, a glial cell in the brain, can be stimulated by IFNγ and TNFα to secrete the inflammatory cytokines, monocyte chemotactic protein 1 (MCP-1), interleukin 6 (IL-6), and interferon-γ inducible protein 10 (IP-10).
“CD8+ T cells can contribute to neuroinflammation by secretion of inflammatory cytokines like interferon γ (IFNγ) and tumor necrosis factor α (TNFα). Astrocytes, a glial cell in the brain, can be stimulated by IFNγ and TNFα to secrete the inflammatory cytokines, monocyte chemotactic protein 1 (MCP-1), interleukin 6 (IL-6), and interferon-γ inducible protein 10 (IP-10).
 “The present study investigated the effect of the lack of CB1 and CB2 receptors in mice ovarian morphology, folliculogenesis, oocyte retrieval, and oocyte maturation and evaluated the use of Δ9-tetrahydrocannabinol (THC) on oocyte in vitro maturation (IVM) by comparing classical IVM and two-step IVM by analyzing the meiotic competence of the oocytes and their evolution toward embryos.
“The present study investigated the effect of the lack of CB1 and CB2 receptors in mice ovarian morphology, folliculogenesis, oocyte retrieval, and oocyte maturation and evaluated the use of Δ9-tetrahydrocannabinol (THC) on oocyte in vitro maturation (IVM) by comparing classical IVM and two-step IVM by analyzing the meiotic competence of the oocytes and their evolution toward embryos. “Potential therapeutic actions of the cannabinoids delta-9-tetrahydrocannabinol (THC) and
“Potential therapeutic actions of the cannabinoids delta-9-tetrahydrocannabinol (THC) and