 “Exemestane is one of the aromatase inhibitors (AI) used as first line treatment for estrogen-receptor positive breast cancer in post-menopausal women. Exemestane acts by inhibiting aromatase, the enzyme responsible for the conversion of androgens to estrogens and also by promoting apoptosis of breast cancer cells. Nevertheless, despite its therapeutic success, this AI, after prolonged treatment, can induce acquired resistance, which causes tumor relapse. Therefore, it is important to find new strategies to overcome resistance in order to improve breast cancer treatment.
“Exemestane is one of the aromatase inhibitors (AI) used as first line treatment for estrogen-receptor positive breast cancer in post-menopausal women. Exemestane acts by inhibiting aromatase, the enzyme responsible for the conversion of androgens to estrogens and also by promoting apoptosis of breast cancer cells. Nevertheless, despite its therapeutic success, this AI, after prolonged treatment, can induce acquired resistance, which causes tumor relapse. Therefore, it is important to find new strategies to overcome resistance in order to improve breast cancer treatment.
Considering that the development of resistance is the main reason for endocrine treatment failure, our group decided to explore the ability of three cannabinoids, Δ9-tetrahydrocannabinol (THC), cannabidiol (CBD) and anandamide (AEA), to reverse resistance to exemestane. The THC and CBD are phytocannabinoids derived from the plant Cannabis sativa (marijuana) whereas AEA is an endocannabinoid. For that, it was used LTEDaro cells, a long-term estrogen deprived ER+ breast cancer cell line that mimics resistance to exemestane. These cells were treated with exemestane in combination with two phytocannabinoids, CBD and THC, and the endocannabinoid AEA.
The presence of CB1 and CB2 in LTEDaro cells was confirmed by Western blot analysis and the effects of the combination of cannabinoids with exemestane were evaluated by MTT and LDH assays. Cell morphology was analyzed by Giemsa and Hoechst staining.
Results: Our results demonstrate that all the cannabinoids induce a decrease in viability of exemestane-resistant cells, in a dose- and time-dependent manner, without LDH release. These results indicate that the studied cannabinoids, mainly THC and AEA, revert the resistance to exemestane, probably by inducing apoptosis, as observed in Giemsa/Hoechst stain by the presence of typical morphological features of apoptosis.
Conclusion: This study highlights the efficacy of using cannabinoids as a potential adjuvant treatment to revert resistance to AIs.”
https://www.ncbi.nlm.nih.gov/pubmed/32258721
https://journals.lww.com/pbj/fulltext/2017/09000/The_effects_of_cannabinoids_in.118.aspx

 “Over the last decades a renewed interest in n-3 very long polyunsaturated fatty acids (PUFAs), derived mainly from fish oils in the human diet, has been observed because of their potential effects against cancer diseases, including breast carcinoma. These n-3 PUFAs mainly consist of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) that, alone or in combination with anticancer agents, induce cell cycle arrest, autophagy, apoptosis, and tumor growth inhibition. A large number of molecular targets of n-3 PUFAs have been identified and multiple mechanisms appear to underlie their antineoplastic activities. Evidence exists that EPA and DHA also elicit anticancer effects by the conversion to their corresponding ethanolamide derivatives in cancer cells, by binding and activation of different receptors and distinct signaling pathways. Other conjugates with serotonin or dopamine have been found to exert anti-inflammatory activities in breast tumor microenvironment, indicating the importance of these compounds as modulators of tumor epithelial/stroma interplay. The objective of this review is to provide a general overview and an update of the current n-3 PUFA derivative research and to highlight intriguing aspects of the potential therapeutic benefits of these low-toxicity compounds in breast cancer treatment and care.”
“Over the last decades a renewed interest in n-3 very long polyunsaturated fatty acids (PUFAs), derived mainly from fish oils in the human diet, has been observed because of their potential effects against cancer diseases, including breast carcinoma. These n-3 PUFAs mainly consist of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) that, alone or in combination with anticancer agents, induce cell cycle arrest, autophagy, apoptosis, and tumor growth inhibition. A large number of molecular targets of n-3 PUFAs have been identified and multiple mechanisms appear to underlie their antineoplastic activities. Evidence exists that EPA and DHA also elicit anticancer effects by the conversion to their corresponding ethanolamide derivatives in cancer cells, by binding and activation of different receptors and distinct signaling pathways. Other conjugates with serotonin or dopamine have been found to exert anti-inflammatory activities in breast tumor microenvironment, indicating the importance of these compounds as modulators of tumor epithelial/stroma interplay. The objective of this review is to provide a general overview and an update of the current n-3 PUFA derivative research and to highlight intriguing aspects of the potential therapeutic benefits of these low-toxicity compounds in breast cancer treatment and care.” “Radiotherapy combined with chemotherapy is the major treatment modality for human glioblastoma multiforme (GBM). GBMs eventually relapse after treatment and the average survival of GBM patients is less than two years.
“Radiotherapy combined with chemotherapy is the major treatment modality for human glioblastoma multiforme (GBM). GBMs eventually relapse after treatment and the average survival of GBM patients is less than two years. “The aim of this review article is to summarize current knowledge about the role of cannabinoids and cannabinoid receptors in tumor disease modulation and to evaluate comprehensively the use of cannabinoids in cancer patients.
“The aim of this review article is to summarize current knowledge about the role of cannabinoids and cannabinoid receptors in tumor disease modulation and to evaluate comprehensively the use of cannabinoids in cancer patients. “According to recent studies,
“According to recent studies, 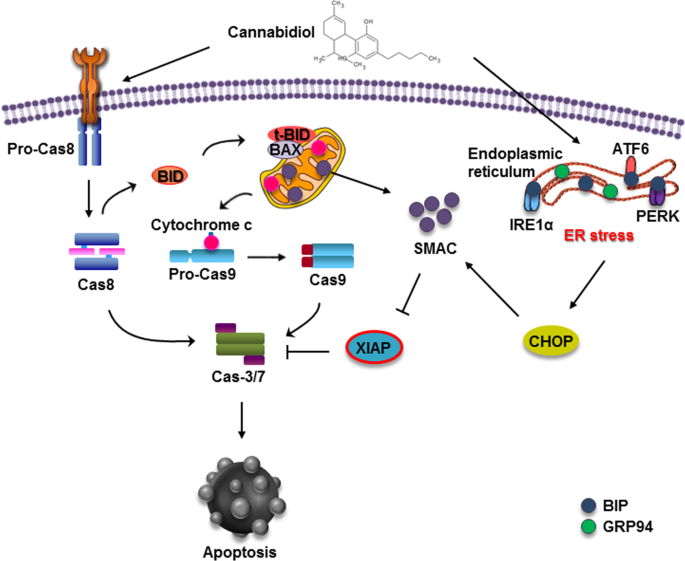
 “The anticancer effects of the omega-3 long chain polyunsaturated fatty acids (LCPUFA), EPA and DHA may be due, at least in part, to conversion to their respective endocannabinoid derivatives, eicosapentaenoyl-ethanolamine (EPEA) and docosahexaenoyl-ethanolamine (DHEA).
“The anticancer effects of the omega-3 long chain polyunsaturated fatty acids (LCPUFA), EPA and DHA may be due, at least in part, to conversion to their respective endocannabinoid derivatives, eicosapentaenoyl-ethanolamine (EPEA) and docosahexaenoyl-ethanolamine (DHEA). “The endogenous lipid metabolism network is associated with the occurrence and progression of malignancies.
“The endogenous lipid metabolism network is associated with the occurrence and progression of malignancies. “Total phenolic content and antioxidant activity of polar extracts of edible resources from Fedora hemp cultivar (Cannabis sativa L.), namely seed, flour and oil, were evaluated. The main components in the polar extracts were identified using HPLC-DAD and HPLC-ESI-MS/MS. As expected, the molecular profile of components from seeds and flour was strictly similar, dominated by N-trans-caffeoyltyramine. The profile of oil polar extracts contained hydroxycinnamic acid derivatives and
“Total phenolic content and antioxidant activity of polar extracts of edible resources from Fedora hemp cultivar (Cannabis sativa L.), namely seed, flour and oil, were evaluated. The main components in the polar extracts were identified using HPLC-DAD and HPLC-ESI-MS/MS. As expected, the molecular profile of components from seeds and flour was strictly similar, dominated by N-trans-caffeoyltyramine. The profile of oil polar extracts contained hydroxycinnamic acid derivatives and  “Kidney ischemia reperfusion (IR) injury is an important health problem resulting in acute renal failure. After IR, the inflammatory and apoptotic process is triggered.
“Kidney ischemia reperfusion (IR) injury is an important health problem resulting in acute renal failure. After IR, the inflammatory and apoptotic process is triggered. “Phytocannabinoids are unique terpenophenolic compounds predominantly produced in the glandular trichomes of the cannabis plant (Cannabis sativa L.). The delta-9- tetrahydrocannabinol (THC) is the main active constituent responsible for the plant’s psychoactive effect and, together with the non- psychoactive cannabidiol (CBD), the most investigated naturally occurring cannabinoid.
“Phytocannabinoids are unique terpenophenolic compounds predominantly produced in the glandular trichomes of the cannabis plant (Cannabis sativa L.). The delta-9- tetrahydrocannabinol (THC) is the main active constituent responsible for the plant’s psychoactive effect and, together with the non- psychoactive cannabidiol (CBD), the most investigated naturally occurring cannabinoid.