 “Background: Various factors trigger the inflammatory response and cytokine activation in skeletal muscle. Inflamed muscle will exhibit significant levels of inflammation and cytokine activity. Interleukin-6 (IL-6), a pro-inflammatory cytokine, exerts pleiotropic effects on skeletal muscle. Endocannabinoid produced by all cell types binds to a class of G protein-coupled receptors, in particular cannabinoid CB1 receptors, to induce skeletal muscle actions.
“Background: Various factors trigger the inflammatory response and cytokine activation in skeletal muscle. Inflamed muscle will exhibit significant levels of inflammation and cytokine activity. Interleukin-6 (IL-6), a pro-inflammatory cytokine, exerts pleiotropic effects on skeletal muscle. Endocannabinoid produced by all cell types binds to a class of G protein-coupled receptors, in particular cannabinoid CB1 receptors, to induce skeletal muscle actions.
Objective: The purpose of this research was to discover whether activation of cannabinoid CB1 receptors in L6 skeletal muscle cells may promote IL-6 gene expression.
Materials and methods: L6 skeletal muscle cells were cultured in 25 cm2 flasks and quantitative reverse transcription-polymerase chain reaction (probe-based) utilised to quantify IL-6 gene expression levels among different treatment settings.
Results: Arachidonyl-2′-chloroethylamide (ACEA) 10 nM, a persistent selective CB1 receptor agonist, promotes IL-6 gene expression in a time-dependent manner. Rimonabant 100 nM, a selective cannabinoid CB1 receptor antagonist, blocks the impact of ACEA. However, insulin does not change IL-6 gene expression.
Conclusion: For the first time, a unique link between ACEA and IL-6 up-regulation has been established; IL-6 up-regulation generated by ACEA is mediated in skeletal muscle through cannabinoid CB1 receptor activation. As a result, cannabinoid CB1 receptors may be useful pharmaceutical targets in the treatment of inflammation and related disorders in skeletal muscle tissues.”
“In the present study, I have demonstrated that when cannabinoid CB1 receptors are activated, the expression of IL-6 increases in a way that is influenced by time. Such findings deliver a novel mechanism characterised by cannabinoid analogue playing the role of a pro-inflammatory mediator in the skeletal muscle tissue. The findings from the present study also imply that there may be a possible therapeutic use of cannabinoid CB1 receptor antagonist at acute early states for skeletal muscle dysfunction related to inflammation. My findings point to skeletal muscle cell cannabinoid CB1 receptor as a therapeutic target, and expand its potential to include anti-inflammatory effects in diabetes, obesity, and sarcopenia.”

 “Cannabidiol is increasingly considered for treatment of a wide range of medical conditions.
“Cannabidiol is increasingly considered for treatment of a wide range of medical conditions.  “Importance:
“Importance:  “Studies investigating the psychosomatic effects of social isolation in animals have shown that one of the physiologic system that gets disrupted by this environment-affective change is the Endocannabinoid System. As the levels of endocannabinoids change in limbic areas and prefrontal cortex during stressful times, so is the subject more prone to fearful and negative thoughts and aggressive behavior. The interplay of social isolation on the hypothalamic-pituitary-adrenal axis and cannabinoid tone triggers a vicious cycle which further impairs the natural body’s homeostatic neuroendocrine levels and provokes a series of risk factors for developing health complications. In this paper, we explore the psychosomatic impact of prolonged quarantine in healthy individuals, and propose management and coping strategies that may improve endocannabinoid tone, such as integration of probiotics, cannabidiol, meditation, and physical exercise interventions with the aim of supporting interpersonal, individual, and professional adherence with COVID-19 emergency public measures whilst minimizing their psycho-physical impact.”
“Studies investigating the psychosomatic effects of social isolation in animals have shown that one of the physiologic system that gets disrupted by this environment-affective change is the Endocannabinoid System. As the levels of endocannabinoids change in limbic areas and prefrontal cortex during stressful times, so is the subject more prone to fearful and negative thoughts and aggressive behavior. The interplay of social isolation on the hypothalamic-pituitary-adrenal axis and cannabinoid tone triggers a vicious cycle which further impairs the natural body’s homeostatic neuroendocrine levels and provokes a series of risk factors for developing health complications. In this paper, we explore the psychosomatic impact of prolonged quarantine in healthy individuals, and propose management and coping strategies that may improve endocannabinoid tone, such as integration of probiotics, cannabidiol, meditation, and physical exercise interventions with the aim of supporting interpersonal, individual, and professional adherence with COVID-19 emergency public measures whilst minimizing their psycho-physical impact.” “Cannabis sativa
“Cannabis sativa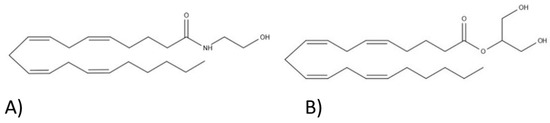
 “The Cannabis sativa plant has been used medicinally and recreationally for thousands of years, but recently only relatively some of its constituents have been identified.
“The Cannabis sativa plant has been used medicinally and recreationally for thousands of years, but recently only relatively some of its constituents have been identified.  “α-Pinene represents a member of the monoterpene class and is highly distributed in higher plants like conifers, Juniper ssp. and Cannabis ssp.
“α-Pinene represents a member of the monoterpene class and is highly distributed in higher plants like conifers, Juniper ssp. and Cannabis ssp. 
 “Introduction: Cannabis sativa L. (C. sativa) is used since ancient times to produce fabrics, baskets, and cords. Later, different ethnic groups used to burn the leaves and flowers of psychotropic cultivars with high Δ9-tetrahydrocannabinol (D9-THC) levels, during the religious or propitiatory rites to alter the state of consciousness. To date, it is not known whether also nonpsychotropic cultivars of C. sativa were used during these rites, and whether these varieties could have an effect on human behavior.
“Introduction: Cannabis sativa L. (C. sativa) is used since ancient times to produce fabrics, baskets, and cords. Later, different ethnic groups used to burn the leaves and flowers of psychotropic cultivars with high Δ9-tetrahydrocannabinol (D9-THC) levels, during the religious or propitiatory rites to alter the state of consciousness. To date, it is not known whether also nonpsychotropic cultivars of C. sativa were used during these rites, and whether these varieties could have an effect on human behavior.